
Full Html
Vol 2 Issue 3 (Special Issue)
Magnetically Modulated for Medical Application: Diagnosis, Drug Delivery, and Therapy
Pages: 101-114
Doi: 10.54738/MI.2022.2305
Doi URL: http://doi.org/10.54738/MI.2022.2305
Ayesha Nawaz 1 , Muhammad Tayyab 1 , Maryam Anwar 1 , Qandeel Khalid 1 , Nadia Shamshad Malik 2 , Ainy Butt 1 , Nayab Tahir 3 , Shamoon Al Islam 4 , Gul Shahnaz 1 , Asadullah Madni , Mubashar Rehman?? 1
1 Department of Pharmacy, QuaidiAzam University, Islamabad, 45320, Pakistan
2 Faculty of Pharmacy, Capital University of Science and Technology, Islamabad, Pakistan
3 College of Pharmacy, University of Sargodha, Sargodha, Pakistan
4 Department of Physics, 5Department of Pharmaceutics, Faculty of Pharmacy, University of Agriculture, Faisalabad, 38000, Pakistan
5 The Islamia University of Bahawalpur, Bahawalpur, 63100, Pakistan
Nanoparticles range in size from 1-100 nm although much larger nanoparticles i.e. up to 300 nm, are widely reported for medical application. Current trends in drug delivery research have shifted focus toward the designing of the “smart” drug delivery systems (DDS) for spacial and temporal control of the drug delivery. When a magnetic moiety is added to a DDS i.e. nanoparticle or liposome, it can be retained in a specific part of the body through localized magnetic field. These magnetically modulated drug delivery systems (MDDS) can also carry payload to deep lying tumor tissues which are difficult to target with other targeting modalities. MDDS are also used as hyperthermic agents under the influence of externally applied alternating magnetic field. Not only the magnetic hyperthermia can kill cancer cells but also causes phase-change in nanoparticles to induce abrupt drug release. Magnetic resonance imaging (MRI) is a diagnostic techniques used to image disease specific changes in tissues using contrast agents such as iron oxide nanoparticles. When iron oxide nanoparticles are loaded with drugs, they act as a contrast agent and carrier for targeted drug delivery which is revolutionizing medical field. In addition to drug delivery applications, magnetic nanoparticles are also being used in biosensors for identification and separation of target molecules/cells from complex mixture. However, challenges associated with optimized particle size, selection of biocompatible materials, and fate of MDDS after in vivo application need to be addressed. Emerging literature also points towards interaction of magnetic field with human body. Thus, carefully tailored magnetic modulated nanoparticles are expected to emerge as a key player in medical field due to their unique diagnostic, therapeutic, sensing and multifunctional application.
Keywords
MRI, Nanoparticles, Liposomes, Hyperthermia, Hydrogels, Thermoresponsive
Current trends in the drug delivery research have shifted focus toward designing the “smart” drug delivery systems (DDS) that can provide spatial and temporal control of drug delivery1. Spatial control of drug delivery is important because many new therapeutic agents are non-specific i.e. they can affect both healthy and disease-affected cells2. This objective has been achieved by devising targeted drug delivery systems that can carry the drug exclusively to the disease-specific tissues. Temporal control of drug delivery systems is required in a situation where delayed or sequential drug release is desirable3. Novel DDS have been employed for diagnostic applications ranging from whole organ imaging to single-cell detection4. Recent developments of dual modalities ensure diagnosis, targeting, and treatment of challenging diseases in a single administration.
The curiosity to identify the medical benefits of the magnetic field started as soon as the discovery of the magnetic field. Although a lot of claims have been made of its potential benefits, scientific proof of such claims is limited or even absent in some cases. In pharmaceutical sciences, the magnetic field has shown promising applications to control the site and rate of release of potent therapeutic agents from DDS by externally applied magnetic field5. Magnetically modulated drug delivery systems (MDDS) have attracted vast interest in medical research due to the many important properties of the magnetic field. First, the magnetic field can easily pass through the body and the magnetic permeability of the human body is roughly the same as that of air6. This provides the opportunity to target MDDS to deep tissues which is a limitation of most other targeting strategies. Second, the magnetic field is generally considered safer than radiations and, currently, there are no clinically proven side effects after short-term application for diagnostic and therapeutic applications7. When a magnetic moiety is added in a DDS i.e. nanoparticle or liposome, it can be retained in a specific part of the body through a localized magnetic field. These MDDS can carry a payload to deep-lying tumor tissues which are difficult to target with other targeting modalities. Magnetic nanoparticles (MNP) are also used as hyperthermic agents under the influence of externally applied magnetic fields to induce drug release from DDS or as thermotherapy to kill cancer cells8-9. Diagnostic applications of MDDS ranges from MRI contrast agents to magnetic cell separation techniques. This review article discusses different applications of magnetically modulated drug delivery systems. Various medical applications that have been enhanced by a magnetic field are summarized in table 1. Magnetic field can be used at three different frequency ranges that are static, time-varying and radio frequency. Due to the phenomenon of coexistence of electrical and magnetic fields, reports have emerged on the unwanted health effects.
Table 1: Diagnostic and treatment applications of magnetically modulated nanocarriers in different medical conditions.
|
Application |
Magnetic system |
Mechanism |
|
Diagnosis |
Magnetic Resonance Imaging (MRI) |
MRI is an imaging technique which measures changes in proton density in target tissues that occur during course of disease. |
|
Magnetic cell separation |
Target cells are tagged with magnetic nanoparticles and magnetic field is applied to separate the tagged cells. |
|
|
Immunoassays |
Antibodies are conjugated to the magnetic nanoparticles and magnetic field is applied to collect nanoparticles bound antibody-target molecule (antigen) complexes. |
|
|
Cancer treatment |
Alternating magnetic field (AMF) for thermotherapy |
Magnetic nanoparticles are given to patient that accumulate in cancer. Then, AMF is applied which produces hyperthermia to kill cancer cells. |
|
MRI for image guided therapy |
This involves administration of magnetic nanoparticles for imaging of cancer using MRI. Then, high frequency AMF is applied to produce hyperthermia, as in thermotherapy, or to induce release of co-loaded drug for treatment. |
|
|
Brain function study |
MRI |
Changes in blood flow during performance of different tasks shows which part of brain is associated with the function. |
|
Drug delivery |
Static magnetic field |
Magnetic nanoparticles are attached to drug delivery systems and an externally applied magnetic field is used to guide their transport in the body. |
|
MRI |
MRI of is used to produces hyperthermia to induce drug release from magnetic nanoparticles tagged drug delivery systems. |
|
|
Psychic diseases |
Transcranial magnetic stimulation (TMS) |
AMF is applied over specific brain point to induce electric pulses as mean to induce or to synchronize neuron firing. |
|
Magnetic seizure therapy (MST) |
AMF of higher frequency than used in TMS is used to cause seizure to control brain function. |
|
|
Musculoskeletal diseases |
Millimeter wave therapy |
Uses AMF of extremely high frequency to treat pain and bone healing. |
|
Microwave diathermy |
Induces localized hyperthermia to relieve pain and swelling, and to improve healing. |
|
|
Pulsed AMF |
Pulses of electromagnetic field reduce degenerative pathways and induce regenerative and growth cells. |
|
|
Static magnetic field |
Guides direction of growth of new cells in tissue engineering |
A magnetic field has been largely used for imaging and cell separation due to lesser side effects than the use of hazardous radiations. It is also replacing surgical procedures to obtain biopsies which is a common method used for the diagnosis of many diseases.
Magnetic Resonance Imaging:
MRI is a non-invasive diagnostic technique that uses a magnetic field to produce a three-dimensional image of different body tissues and organs. MRI operation involves placing the patient in the strong magnetic field that will make protons in mobile water of the body, usually hydrogen nucleus, to align along with the applied field. Then, a radiofrequency pulse is passed through the body that will stimulate the protons to pull out of the applied field. When the radiofrequency source is removed, protons revert to realign to a normal state and release energy10. The time protons take to realign with the magnetic field and the amount of energy released are characteristic of the anatomical environment and chemical nature of tissues. Due to the absence of ionizing radiations, MRI is considered superior to CT scan and X-ray-based imaging techniques especially when repeated imaging is required11. MRI has also effectively replaced invasive procedures to obtain biopsies in many diagnostic applications. In addition, MRI is more suitable for soft tissue imaging than other imaging modalities12. Native contrast in MRI imaging is proton density, T1 relaxation (recovery of longitudinal magnetization), and T2 relaxation (recovery of transverse magnetization).13 However, contrast-enhancing agents may be given to the patient before MRI to facilitate the faster realignment of protons and produce a brighter image.14 These contrast agents may act at any sub-atomic event in the magnetic resonance mechanism. Iron oxide nanoparticles have emerged as excellent contrast agents in cancer detection with added benefits of their theranostic activity.15 By controlling magnetic field and radiofrequency pulse, a wide variety of pathologies can be diagnosed due to changes occurring in proton density during the disease. The first step in MNP enhanced MRI is the systemic injection for site-specific accumulation by application of magnetic field or by attachment of targeting ligand16. Then, MRI is performed for high-quality imaging (figure 1). MNP coating with antifouling agents prevents surface attachment of proteins and other biological moieties that can limit contrast efficiency in the body17. MRI has also been used in combination with drug-loaded nanoparticles for image-guided drug delivery. Similarly, MRI has been used to induce hyperthermia from MNP which will be discussed in the next headings.
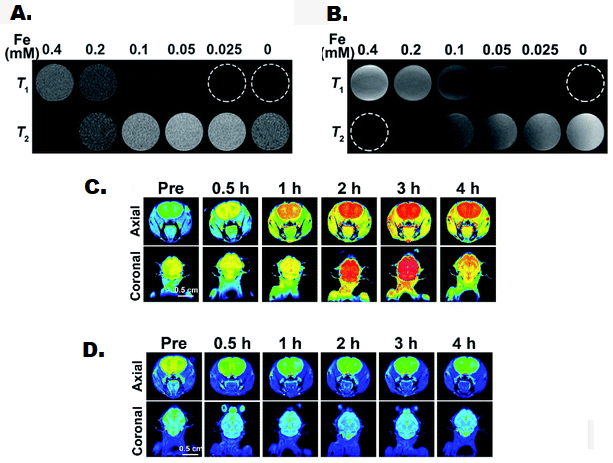
Figure 1: Presentation of small sized iron oxide nanoparticles as contrast for MRI imaging of brain. T1 and T2 weighted phantom imaging of iron oxide nanoparticles was acquired at 0.5 T (A) and 1.5 T (B) scanner. T1 (C) and T2 (D) weighted imaging of nude mice after intravenous injection of iron oxide nanoparticles at the dose of 2 mg iron per kg of body weight. Adapted from ref. 16. Copyright of Royal Society of Chemistry, 2021.
Magnetic separation techniques:
The magnetic field’s ability to control the movement of magnetic nanoparticles in time and space has innovated the field of separation techniques, as following:
Magnetic cell separation techniques: The separation of the magnetic and nonmagnetic components in a mixture is made possible by the application of a magnetic field as a driving force. However, biomedical applications have emerged in the last few decades18. The isolation and purification of different cells and biochemical molecules have been recognized as the most important application of MNP19. In magnetic cell separation, cell sorting is done through the attraction of labeled cells in a heterogeneous mixture towards magnetic flux. The first step is to label MNP with specific ligands for target cells to be separated. Labeled MNP is incubated with cell culture for a specified time to allow targeted binding on the cell surface. This can be facilitated by using a positively charged polymer matrix that can bind negatively charged cell membranes20. The interactions mediated by targeted ligands reduce the time for adsorption and enhance the separation efficiency of different components. Core-shell microspheres formed by using the modified silica and Fe3O4 as a magnetizing agent have also been used for application in the separation of cells and different biomolecules such as nucleic acid.21 Aldehyde modified silica nanoparticles have demonstrated better adsorptive and targeting properties in in vitro experiments.22
Magnetic nanoparticles in immunoassay: The magnetic field can also be used to assist immunoassay-based separation techniques. This is done by attaching the antibody to MNP for the detection of target biological molecules. The magnetic field is used in these systems to aid in the detection and separation of bound molecules. Interestingly, MNP can be recovered after the experiment as reusable agents.23-24
Magnetic nanoparticles in biosensors: MNP has been engineered as biosensors for one-step detection of various analytes. These types of sensors can measure changes in signal, such as light reflectance and electrical resistance, due to the conjugation of MNP with the biological analyte. The MNP is paving the way for fabrications of highly sensitive, rapid, and economic biosensors for mobile applications.
Lack of selectivity and uneven distribution of different therapeutic agents has limited the use of many potent chemotherapeutic agents. Targeted DDS are designed to deliver drugs selectively to the desired site. The targeted delivery of DDS by conjugation with magnetic nanoparticles has opened a new era in drug delivery25. MDDS target loaded drugs or other therapeutic moieties to the desired site by an externally applied static magnetic field. On the other hand, MNP has been used to initiate drug release from colloidal DDS due to hyperthermia induced by an alternating magnetic field. Multiple systems, depending upon their size, functional capabilities, and structural composition, have been categorized under magnetic carriers including magnetic liposome, noisome, micro nanoparticles.
Magnetic microparticles and nanoparticles
Magnetic microparticles tend to respond to the static magnetic field by moving in direction of the field lines according to Coulomb’s Law.26 Magnetic microparticles can respond to alternating magnetic fields leading to the transition of energy from the field to the microparticles. The resulting heating may be used for hyperthermia therapy and to aid in the release of potent drugs from thermoresponsive DDS.27 Magnetic particles can be prepared with the flexibility of size range from a few nanometers (usually >10 nm) to tens of micrometers which is comparable to the cell size of (10-20 µm), viruses (0.02-0.45 nm), proteins (5-50 nm), and gene products (2 µm width and 0.1 nm length). So, the proximity with biological targets offers a variety of advantages in drug delivery.28 Drug delivery to the central nervous system (CNS) via the blood-brain barrier provides limited access because of physiological differences of blood capillaries in CNS, less solubility, and poor bio-distribution of drug molecules.29 Conventional delivery of chemotherapeutic agents had shown to be less effective, although the disruption of blood-brain barrier integrity has shown to increase the penetration of drugs during multiple disorders. The development of micro-sized drug carriers (microcapsules) coated with the drug molecule leads to enhanced drug delivery to brain tissues that is further strengthened by the use of magnetic resonance techniques.30 The transport of magnetic microcapsules in the blood vessels was demonstrated by different biomimetic approaches. These studies elaborate on the effect of blood flow and particle size as well as other electrostatic and steric forces.31 Furthermore, magnetically modulated targeting of microparticles to brain tissues does not induce an immune response which has been a limiting factor for many novel DDS.
MNP is a class of magnetic particles in the size range of 1-100 nm. Advancement in the availability of different biodegradable, as well as non-biodegradable materials and developmental technologies, facilitate the production of these particles with different physical, chemical, and functional modalities. MNP decorated with different functional modalities has been utilized to address the above-mentioned challenges of diagnosis and treatment of the different diseases in spatiotemporal mode32. MNP can be retained at localized tissue due to externally applied magnetic fields. One example of these systems is the high retention of nanoparticles at inflamed tissues of the skin and the underlying musculoskeletal system. After the drug is released, the magnetic field can be switched off leading to the elimination of nanoparticles from the body.33 Another targeting application of magnetic field is locoregional chemotherapy. This involves intra-arterial administration of nanoparticles that are directed to and localized in tumor tissue by an externally applied static magnetic field. This increases drug accumulation in targeted tissue leading to improve efficacy.34 Recently, magnetosomes have gained interest in the medical field due to their biocompatible nature. These are intracellular nanoparticles chain used by magnetotactic bacteria to navigate in the direction of the earth’s magnetic field. Due to their homogenous size and vesicular structure, they have been used for the delivery of large drug molecules. Gareev et al. has written a review article that comprehensively discusses magnetosome applications in the medical field.35 MNP has also been used to induce drug release from other drug delivery systems. This goal is achieved usually by bonding a magnetic moiety to a polymer resulting in an amphiphilic structure of polymer that can form nanoparticles by self-assembly in vitro or in vivo. Conversely, MNP can be loaded in as-formed polymeric nanoparticles to cause thermoresponsive drug release at the target site 32b 36, 37. The advantages in two ways i.e.amount disease-affected target tissuesirpharmacological effect normal drugtoxic Thegelatin capsule to GIT, thus, releasing the encapsulated drug at the target site In another study, functionalized montmorillonite (MMT) materials were used as unique porous structures(FePt)FePt@MMT contract enhancer FePt@MMT-MIT, in addition to MRI imaging and MFH, chemotherapy38. Similarly, hybrid systems have been prepared for simultaneous delivery of chemotherapeutic agents, gene products, and SPION, a strategy that may enable complete eradication of tumor and associated stem cells. Another recent innovation in magnetically modulated drug delivery is nanomotors, sometimes referred to as nano-swimmers that can swim in the blood to deliver encapsulated drugs to the target site. These nanomotors rotate under the influence of the applied magnetic field and move toward the target, thus no build-in fuel reservoir is needed. Another benefit of nanomotors is their ease to cross biological barriers by disrupting the target membrane or extracellular matrix.
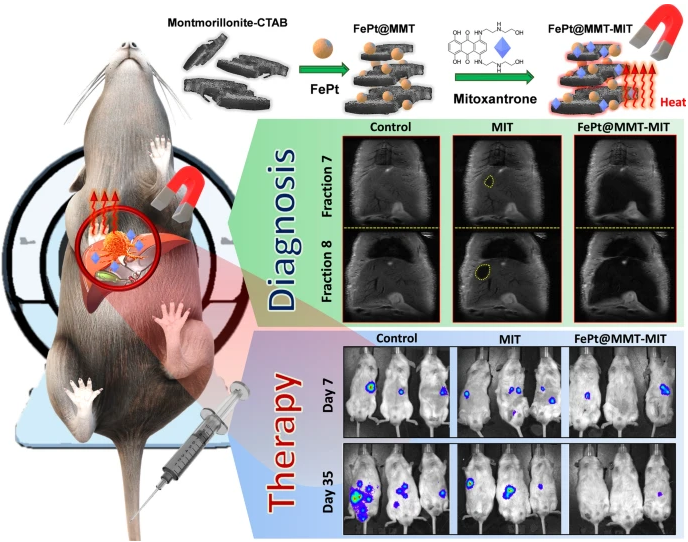
Figure 2: Mitoxantrone loaded FePt nanoparticles for diagnosis, magnetic hyperthermia (thermotherapy), and targeted chemotherapy of cancer in mice. Adopted from ref. 38. Copyright of Springer, 2021.
Magnetic liposome
Liposomes consist of a phospholipid bilayer and an internal aqueous core which enable them to encapsulate both hydrophobic and hydrophilic drugs, respectively. Advances in liposomal drug delivery involve targeting and “clickable” drug release by the inclusion of magnetic or paramagnetic particles39- 41. The magnetic particles can be incorporated in the central core of vesicles, lipid bilayer, or make complexes on the surface of the liposome (figure 3). In addition, phospholipids tagged with magnetic or paramagnetic materials such as gadolinium can be used to form liposomes. The presence of magnetic materials tends to align the movement of liposomes along the lines of the magnetic field and enhance the penetrability and penetration of liposomes at the target site. Drug release from liposomes is mediated by magnetically induced hyperthermia. When magnetic liposomes are exposed to an alternating magnetic field, hyperthermia is produced which destabilizes lipid bilayer leading to the release of encapsulated drug. Consequently, the drug release rate can be controlled by varying patterns or strength of applied magnetic field42. Fabrication of magnetic liposomes with targeting ligands, functional groups, fluorescent compounds, and contrasting agents enables multi-modal applications in diagnosis, imaging, and delivery of multiple therapeutic compounds to the target cell and tissues.1, 43 The most important application of magnetic liposomes is the delivery of gene products due to their intrinsically high penetration in cells and transfection efficiency. Cationic lipids are generally used for these applications because they can strongly bind negatively charged nucleic acid.44
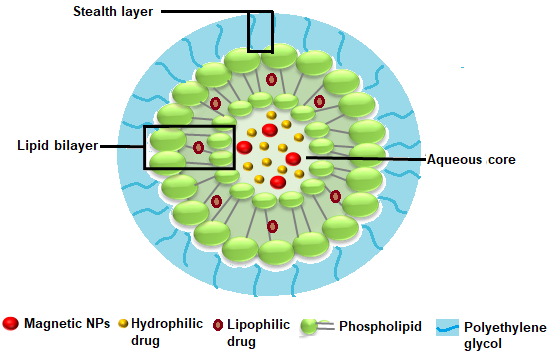
Figure 3: Structure of magnetic liposome consisting of a phospholipid bilayer coating an aqueous core containing magnetic NPs and drug payload.
Magnetic Hydrogels
Hydrogels are a three-dimensional network of cross-linked polymers with tunable characteristics such as versatile chemical nature and biocompatibility.45 The hydrogels can be loaded with different therapeutic and diagnostic modalities including microparticles, nanoparticles, liposomes, fluorescent and contrasting agents that have been discussed previously (figure 4). Different methods have been employed for the fabrication of these formulations but grafting-onto method46, in situ precipitation method47, and blending methods48 are of prime importance. The incorporation of magnetic particles in the matrix of conventional hydrogels permits greater control over the release profile of different macromolecular therapeutic moieties including peptides, proteins, and hormones. The therapeutic payload, either drug or drug-loaded DDS, is loaded into hydrogel-MNP composite and magnetic hyperthermia is used to induce the release of payload. Hydrogels were also prepared with micron-sized pores for drug loading and subsequent release in a three-dimensional intracellular environment49. Interestingly, pulsatile release of payload can also be achieved by repetitive on and off application of the magnetic field application.50
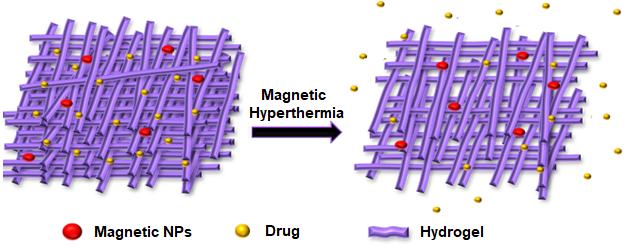
Figure 4: Schematic illustration of drug and magnetic NPs loaded magnetic hydrogel’s network and induction ofdrug release upon magnetic hyperthermia.
Hyperthermia has been used historically to cure illnesses and was a commendable point of interest. It has been assumed that cancer can be treated as well as its growth can be suppressed by fever51. In accordance with this assumption, scientists began to utilize this concept of fever induction to cure cancer52 or using external sources to provide hyperthermia53. To achieve this aim, hyperthermia was induced by many tactics inside the body as well as from outside means via infrared radiation, alternating magnetic fields and ultrasound waves. The primary focus of this review are magnetic field related techniques which utilize heat to cure cancer. Owing to non-toxic character of iron oxide nanoparticles (magnetite Fe3O4 as well as maghemite γ-Fe2O3) have been majorly employed (Figure 5) 54. Type of MNP, magnetosomes, are products of magneto-tactic bacteria inside bacterial cell wall via mineralization of oxides or sulfides of iron, which produce superior results as compared to iron oxide nanoparticles. Every magneto-tactic specie give its peculiar crystals.55 Majority of studies have revealed undesirable experience upon MNP injection into tumor site directly. Currently, tumor-specific ligands have been employed to deliver MNP via IV route. For instance, attempts have been made to target intravenously administered MNP to tumors using tumor-specific ligands. For example, single-core magnetic nanoparticles were covalently conjugated with a fluorochrome and either ant-α-tubulin or anti-β-catenin antibodies for intracellular targeting in mice. Binding of antibodies did not alter the magnetic properties of the MNP. The antibodies retained their immunochemical properties, target specificity, the ability to visualize the protein distribution.56 In case of magnetic field hyperthermia (MFH), there is external application of alternating magnetic field to heat up accumulated MNP inside tumor. The prominent disadvantage of hyperthermia is immediate buildup of heat shock proteins (HSP) upon MFH application. HSP belongs to those kind of proteins that hinder protein aggregation originated by heat heading to thermotolerance.57 Hence, thermotolerance not only has put MFH status at risk as an antitumor therapeutic tool but also restrict its use to once or twice in a week. MFH heating range determines killing pattern of tumor cells such as autophagy, necrosis, and apoptosis.58 Hyperthermia can be applied both locally as well as to the whole body. Higher temperature is required for local hyperthermia rather than whole-body hyperthermia which needs mild rise in body temperature. Classical or sub-lethal MFH has been generated from 41-46°C does not destroy cancer cells.59 Classical MFH gives dose dependent effects which are reversible. Though its unambiguous target is unclear, but it is attributed to impair various intracellular enzymes, receptors, and proteins. Hence it is utilized along with radiotherapy60 as well as chemotherapy.61 Synergism of magnetic field is seen with other modes as well, but it is dependent on application time. Thermo-ablation or lethal MFH has been generated at 55°C, which destroys cancer cells directly.62 This process burns cells due to temperature and results in necrosis at a broad spectrum. One research showed that patients incompliant to traditional chemotherapy, responded well to 43°C hyperthermia without significant adverse effects.63-68 Additionally, it helps to measure extent of tumor mass reduction after MFH application. MFH can regulate flow of blood towards tumor for enhancing approach of immune elements to tumor.
According to recent research it has been seen that against tumors, MFH can produce and enhance the effect of Immune system. It is believed that the initiation of MFH is performed by none other than the HSP family members such as HSP-96 AND HSP-70.68 The HSP-96 Vaccine, which is tumor derived, has been tested previously and it showed good results as in case of antitumor activity.69 Anyhow, the synthesis produced by MSH and HSP release appeared to be an easy option because of its low cost and unavailability of surgical extraction. The altered immune response in sublethal MFH was observed due to higher level of HSP expression which were usually found in suppressed form in the tumors. In case of lethal MFH, release of stored HSP in tumor cell is the reason. Immune response seems appealing for three prominent reasons. First of all, it can upgrade the tumor killing mechanism of body naturally which also lowers the demand of the chemotherapeutic agents.68 Secondly, the modulations in immune system has been seen to kill metastasized tumors that are distant and far away from hyperthermia exposure.65 Third, the recurrent tumor risk decreases as MFH induced immune response lasts for a longer time frame.70 Modified tumor microenvironment and immunosuppressive cells determine HSPs vaccination effect. MFH application to eliminate tumors also counteract casual working of various immune system components in a complicated way. Previously, MFH has also been employed along with MRI as role of contrast agents was played by MNP in MRI.71 This modality opens door to visualize tumor after injecting MNP inside patient body followed by MFH killing of tumor.
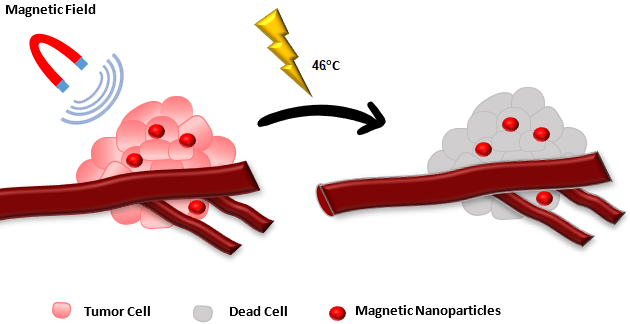
Figure 5: MNP mediated tumor cell destruction under application of alternating magnetic field (AMF). First, nanoparticles are targeted to tumor by application of AMF. Then, frequency of AMF is changed to induce localized tumor ablation
MNP has been traditionally used along with horseradish peroxidase (HRP), an enzyme commonly used for peroxidase activity. In these applications, catalytic activity is carried out by HRP, and MNP is used for magnetically modulated separation of a conjugated system for renewable applications. They can enhance efficiency at different processing steps and biological activity in complex mixture by the application of the magnetic field. However, Vallabani et al. found that MNP, such as magnetite (Fe3O4), possess intrinsic peroxidase-like-activity i.e. catalyze the oxidation of biological materials in the mixture.72 This allows catalysis with Fe3O4 nanoparticles where catalyst can be recovered by a magnetic field at the end or predetermined point. Since then, many inorganic nanoparticles have been tested for enzyme-like activities.72,73 The mechanism of the peroxidase-like activity of MNP is similar to HRP but offers many advantages. The catalytic activity of MNP is directly proportional to the amount of Fe2+ ions on the surface. The presence of a large number of Fe2+ ions on the MNP surface leads to a much higher catalysis rate as compared to HRP which has only one Fe2+ ion. Optimum catalysis activity of MNP, just like HRP, is achieved in a range of H2O2 concentrations above or below which decreases the catalysis rate. Similarly, MNP activity is dependent upon the pH and temperature of the reaction mixture although they have a broader range of these working conditions. Due to their inorganic nature, MNP is much more stable and robust in biological systems. Interestingly, MNP show enzyme activity over a broad size range which makes them preferred candidates for immunoassays, biosensors, and nanodevices.74-75 Most researchers have prepared biosensors and biocatalyst of Fe3O4 in conjugation with other materials. Fe3O4 is conjugated to reduced graphene oxide (rGO) to combine the properties of both materials. rGO is a single atom thick layer of carbon and possesses excellent mechanical strength and flexibility. It has shown mild peroxidase-like activity of its own and can bind almost all materials due to its functionalized surface. Fe3O4-rGO shows higher enzyme activity than Fe3O4 or go alone and can be recovered under magnetic field.72 In this way, it is also possible to stop the catalytic reaction at a certain time to obtain a product of desired composition or associated properties. Together with the physical properties of rGO, the Fe3O4-rGO has emerged as a first-choice inorganic catalyst for economic, robust, reusable, and high sensitivity devices. The Fe3O4 based composites have been successfully evaluated for the detection of various biological compounds such as glucose76, dopamine77 glycoproteins78, etc.
Magnetic field application in the biomedical field is generally regarded as safe. As scientists believe that MF can be used for the treatment of diseases, they should expect the opposite too. Just like chemotherapeutic drugs, uncontrolled exposure to otherwise beneficial magnetic fields may be harmful. An increasing number of studies support the notion that magnetic fields can produce many unwanted effects at frequency and amplitude being used for different biomedical applications. Although systematic reviews and meta-analysis studies have rejected some of these studies based on improper design or lack of statistically significant results, all of these findings cannot be ignored. In the case of MRI, many problems were reported including attraction of metallic objects in the room, interference with electromagnetic or metallic medical devices used by the patient, and unwanted side effects especially in patients with previous history.79 During the initial years of MRI, a few accidental injuries and even deaths were reported due to metallic objects, such as oxygen cylinders or chairs, being attracted by the strong magnetic field and hitting the patient in MRI machine.80 MRI interaction with metallic devices or implants used by the patients is two-tier. First, the static magnetic field can strongly attract metallic devices. Second, alternating AMF can generate current in these devices which may lead to fatal consequences. These effects can be hazardous to patients and may also distort image quality. However, current clinical practices have introduced various precautions and safety measures to reduce such complications. Usually, prior counseling of patients about the basics safety and efficacy of MRI is a useful step to prepare them for MRI and feel comfortable during the examination. Electromagnetic fields have been studied widely in animals and humans for their carcinogenic and genotoxic effects. The international agency of cancer research has categorized extremely low-frequency magnetic fields (ELF) (3-300 Hz) as possible carcinogens (class 2B). Currently, childhood leukemia is the only type of carcinogenicity whose association with occupational exposure to ELF is supported by scientific evidence.81 Direct damage to genes is not supported by well-designed studies, however, such effects may be observed in the presence of genotoxic agents.82 Similarly, various studies conducted to evaluate the effect of the static magnetic field indicated an increased risk of genotoxic effect. The genotoxic effects appeared in a dose-dependent fashion and tend to disappear after the removal of the magnetic field.83 On the other hand, Gunes et al. reported that genotoxic effects are negligible even at the stronger magnetic fields used in clinical settings.84
Association between behavioral changes and radiofrequency field has been reported by patients for a long time and termed as electromagnetic hypersensitivity syndrome EHS). However, only a few studies have supported this assumption. Domotor et al. observed that patients who report EHS have a higher score of somatic and psychic traits which is an indicator of lower mental well-being.85 Studies evaluating behavioral aspects showed that ELF improve the memory of participants in one study and impaired it in another study. In a study, sham versus static magnetic field settings was tested on rats to assess behavioral changes. The rats were trained to climb up the hole of the MRI machine to get the food. They found that rats stopped getting food from the hole whenever a magnetic field was applied to it. Nevertheless, this behavior was abolished when sensory parts of the brain were surgically removed.86 Although most studies utilized exposure to random or occupational exposure, the risk may persist with deliberate exposure to medical ELF and static magnetic field (SMF). As discussed in the previous headings, magnetic fields of similar amplitudes or frequencies are being investigated for biomedical applications. Towards the end of the 20th century, emerging literature showed that conscious experience of brain function was related to the synchronization of neurons and not the number of neurons firing. Recently, the conscious electromagnetic information (CEMI) theory of McFadden and Peckett proposed that information from neurons is integrated to form an amplified electromagnetic field which influences the brain’s overall AMF more effectively than would be possible by unsynchronized firing.87 Moreover, some studies have suggested that the heart also produces a magnetic field due to electrical pulses of Purkinje fibers which may interact with different body tissues including the brain.88 These magnetic fields may provide a clue of various biological effects of the magnetic field that are still unexplained. Biomedical application of the magnetic field may tend to interact with such fields and lead to an altered response, which can be either positive or negative.
As a safety measure, it is customary to question the patients about implants, metallic object, condition of vital organs, and gender-specific aspects of health to ensure before MRI examination.89 The same principal should be extended to any medical applications involving magnetic field. In addition to questions mentioned above, the patients should also be inquired about his mental health and cognition if head region is under investigation, to avoid unnecessary negative effects on health. Therefore, the authors stress that well-designed studies are needed to establish the safety of such paradigms both in vitro and in vivo. Precautions and safety measures should also be adopted to ensure the safety of patients and healthcare workers in clinical settings.
Magnetic nanocarriers have found diverse applications in the medical field ranging from drug delivery to drug-free treatment and diagnostic applications. The ability of the MNPs to align with the applied magnetic field has enabled researchers to control the transport of a variety of nanoparticles filled with chemotherapeutics which enhances therapeutic effects and reduces side effects. Under an AMF, MNP produce hyperthermia either to cause ablation or to cause subcellular damage leading to apoptosis. MFH also increases blood flow in tumor and enhances MNPs accumulation. At the same time, MNPs can be loaded in liposomes and hydrogels to degrade the carrier matrix under MFH ad release the payload at targeted site. MNP are conjugated with targeting ligands for site-specific accumulation in the body.
Ligand conjugated magnetic nanoparticles are also used for the separation of cells from a heterogeneous mixture of biological macromolecules in an immunoassay. Similarly, magnetic nanoparticles conjugated to a catalytic enzyme and allow the removal of the enzyme at any stage of the reaction. In addition to contrast enhancement in MRI, the flexibility to control pattern and frequency of MRI has provided an opportunity to target magnetic nanoparticles to target tissue, do diagnostic imaging and to induce hyperthermia after a single administration of magnetic nanoparticles.
Although generally regarded as safe for the human body, emerging literature pointed out the risk of cancer and a behavioral disease after application of magnetic field. Extremely low frequency AMF has been associated with childhood leukemia. Therefore, care must be taken to restrict future research to the range of the magnetic field which is safe for the body. Indeed, the future of medical applications of the magnetic field lies in the engineering of multifarious nanocarriers that will allow simultaneous diagnosis, thermotherapy, targeted drug delivery, or image-guided surgery of diseases.
Madni, Asadullah, Sarfraz, Muhammad, Rehman, Mubashar, Ahmad, Mahmood, Akhtar, Naveed, Ahmad, Saeed, Tahir, Nayab, Ijaz, Shakeel, Al-Kassas, Raida & Löbenberg, Raimar . 2014. Liposomal Drug Delivery: A Versatile Platform for Challenging Clinical Applications. Journal of Pharmacy & Pharmaceutical Sciences 17(3):401.
Kwon, Il Keun, Lee, Sang Cheon, Han, Bumsoo & Park, Kinam . 2012. Analysis on the current status of targeted drug delivery to tumors. Journal of Controlled Release 164(2):108–114.
Mura, Simona, Nicolas, Julien & Couvreur, Patrick . 2013. Stimuli-responsive nanocarriers for drug delivery. Nature Materials 12(11):991–1003.
Emerich, D F . 2005. Nanomedicine – prospective therapeutic and diagnostic applications. Expert Opinion on Biological Therapy 5(1):1–5.
Tietze, Rainer, Lyer, Stefan, Dürr, Stephan, Struffert, Tobias, Engelhorn, Tobias, Schwarz, Marc, Eckert, Elisabeth, Göen, Thomas, Vasylyev, Serhiy, Peukert, Wolfgang, Wiekhorst, Frank, Trahms, Lutz, Dörfler, Arnd & Alexiou, Christoph . 2013. Efficient drug-delivery using magnetic nanoparticles — biodistribution and therapeutic effects in tumour bearing rabbits. Nanomedicine: Nanotechnology, Biology and Medicine 9(7):961–971.
Stavroulakis, P . 2013. Biological effects of electromagnetic fields: Mechanisms, modeling, biological effects, therapeutic effects, international standards, exposure criteria. Springer Science & Business Media
Heinrich, Angela, Szostek, Anne, Meyer, Patric, Nees, Frauke, Rauschenberg, Jaane, Gröbner, Jens, Gilles, Maria, Paslakis, Georgios, Deuschle, Michael, Semmler, Wolfhard & Flor, Herta . 2013. Cognition and Sensation in Very High Static Magnetic Fields: A Randomized Case-Crossover Study with Different Field Strengths. Radiology 266(1):236–245.
Priester, Marjolein I, Curto, Sergio, Rhoon, Gerard C Van & Hagen, Timo L M Ten . External Basic Hyperthermia Devices for Preclinical Studies in Small Animals. Cancers 13(18):4628.
Keevil, S F, Gedroyc, W, Gowland, P, Hill, D L G, Leach, M O, Ludman, C N, Mcleish, K, Mcrobbie, D W, Razavi, R S & Young, I R . 2005. Electromagnetic field exposure limitation and the future of MRI. The British Journal of Radiology 78(935):973.
Berger, A . 2002. How does it work?: Magnetic resonance imaging. BMJ 324(7328):35.
Iannarelli, A, Badia, S, Rengo, M & Mri, . 2018. Diagnostic Imaging in pediatric thoracic trauma. La radiologia medica 75–89.
Luokkala, Toni, Temperley, David, Basu, Subhasis, Karjalainen, Teemu V & Watts, Adam C . 2019. Analysis of magnetic resonance imaging–confirmed soft tissue injury pattern in simple elbow dislocations. Journal of Shoulder and Elbow Surgery 28(2):341–348.
Deoni, S C, Meyers, S M & Kolind, S H . 2015. Modern methods for accurate T1, T2, and proton density MRI. Oxford Textbook of Neuroimaging. (pp. 13)
Shokrollahi, H . 2013. Contrast agents for MRI. Materials Science and Engineering: C 33(8):4485–4497.
Zhang, X.-D, Zhang, L.-J, Wu, S.-Y, Lu, G.-M, Maggioni, D, Arosio, P, Orsini, F, Ferretti, A M, Orlando, T, Manfredi, A, Ranucci, E, Ferruti, P, D'alfonso, G & Lascialfari, A . 2014. Superparamagnetic iron oxide nanoparticles stabilized by a poly (amidoamine)-rhenium complex as potential theranostic probe. Dalton Transactions 20(32):1172–1183.
Ruixue, Wei,, Liu, Yang, Gao, Jinhao, Yong, V. Wee & Xue, Mengzhou . 2021. Small functionalized iron oxide nanoparticles for dual brain magnetic resonance imaging and fluorescence imaging. RSC adavnces 21(11):12867–12875.
Lee, Haerim, Lee, Eunhye, Kim, Do Kyung, Jang, Nam Kyu, Jeong, Yong Yeon & Jon, Sangyong . 2006. Antibiofouling Polymer-Coated Superparamagnetic Iron Oxide Nanoparticles as Potential Magnetic Resonance Contrast Agents for in Vivo Cancer Imaging. Journal of the American Chemical Society 128(22):7383–7389.
Häfeli, U, Schütt, W, Teller, J & Zborowski, M . 2013. Scientific and clinical applications of magnetic carriers. Springer Science & Business Media
Saiyed, Z, Telang, S & Ramchand, C . 2003. Application of magnetic techniques in the field of drug discovery and biomedicine. BioMagnetic Research and Technology 1(1):1.
Safarik, I & Safarikova, M . 2004. Magnetic techniques for the isolation and purification of proteins and peptides. BioMagnetic Research and Technology 2(1):1.
Ma, Wan-Fu, Zhang, Ying, Li, Lu-Lu, You, Li-Jun, Zhang, Peng, Zhang, Yu-Ting, Li, Ju-Mei, Yu, Meng, Guo, Jia, Lu, Hao-Jie & Wang, Chang-Chun . 2012. Tailor-Made Magnetic Fe3O4@mTiO2 Microspheres with a Tunable Mesoporous Anatase Shell for Highly Selective and Effective Enrichment of Phosphopeptides. ACS Nano 6(4):3179–3188.
Gao, Ruixia, Kong, Xuan, Wang, Xin, He, Xiwen, Chen, Langxing & Zhang, Yukui . 2011. Preparation and characterization of uniformly sized molecularly imprinted polymers functionalized with core–shell magnetic nanoparticles for the recognition and enrichment of protein. Journal of Materials Chemistry 21(44):17863.
Arruebo, M, Fernández-Pacheco, R, Ibarra, M R, Santamaría, J, Noguchi, K, Sakakibara, T, Yoshida, T & Enpuku, K . 2007. Biological immunoassay utilizing magnetoresistive sensor and magnetic-field-controlled binding between magnetic markers and targets. IEEE Magnetics Conference (INTERMAG) 2(3):1.
Bruls, D M, Evers, T H, Kahlman, J A H, Lankvelt, P J W Van, Ovsyanko, M, Pelssers, E G M, Schleipen, J J H B, Theije, F K De, Verschuren, C A, Wijk, T Van Der, Zon, J B A Van, Dittmer, W U, Immink, A H J, Nieuwenhuis, J H & Prins, M W J . 2009. Rapid integrated biosensor for multiplexed immunoassays based on actuated magnetic nanoparticles. Lab on a Chip 9(24):3504.
Alexiou, Christoph, Schmid, Roswitha J, Jurgons, Roland, Kremer, Marcus, Wanner, Gerhard, Bergemann, Christian, Huenges, Ernst, Nawroth, Thomas, Arnold, Wolfgang & Parak, Fritz G . 2006. Targeting cancer cells: magnetic nanoparticles as drug carriers. European Biophysics Journal 35(5):446–450.
Ruiz-Hernández, E, López-Noriega, A, Arcos, D & Vallet-Regí, M . 2008. Mesoporous magnetic microspheres for drug targeting. Solid State Sciences 10(4):421–426.
Lévy, M, Wilhelm, C, Siaugue, J.-M, Horner, O, Bacri, J.-C & Gazeau, F . 2008. Magnetically induced hyperthermia: size-dependent heating power of γ-Fe2O3 nanoparticles. Journal of Physics: Condensed Matter 20(20):204133.
Mcbain, S C, Yiu, H H & Dobson, J . 2008. Magnetic nanoparticles for gene and drug delivery. International journal of nanomedicine 3(2):169.
Hamdi, Mustapha & Ferreira, Antoine . 2014. Guidelines for the Design of Magnetic Nanorobots to Cross the Blood–Brain Barrier. IEEE Transactions on Robotics 30(1):81–92.
Devineni, Damayanthi, Klein-Szanto, Andres & Gallo, James M . 1995. Tissue distribution of methotrexate following administration as a solution and as a magnetic microsphere conjugate in rats bearing brain tumors. Journal of Neuro-Oncology 24(2):143–152.
Mathieu, J-B, Beaudoin, G & Martel, S . 2006. Method of Propulsion of a Ferromagnetic Core in the Cardiovascular System Through Magnetic Gradients Generated by an MRI System. IEEE Transactions on Biomedical Engineering 53(2):292–299.
Muñoz-Gámez, J A, Viota, J, Barrientos, A, Carazo, Á, Sanjuán-Nuñez, L, Quiles-Perez, R, Muñoz-De-Rueda, P, Delgado, Á, Ruiz-Extremera, Á, Salmerón, J, Kossatz, S, Grandke, J, Couleaud, P, Latorre, A, Aires, A, Crosbie-Staunton, K, Ludwig, R, Dähring, H, Ettelt, V, Lazaro-Carrillo, A, Saatchi, K, Tod, S E, Leung, D, Nicholson, K E, Andreu, I, Buchwalder, C, Schmitt, V, Häfeli, U O & Gray, S L . 2015. Efficient treatment of breast cancer xenografts with multifunctionalized iron oxide nanoparticles combining magnetic hyperthermia and anti-cancer drug delivery. Nanomedicine: Nanotechnology, Biology and Medicine 35(4):1430–1441.
Ebner, Armin D, Ploehn, Harry J & Ritter, James A . 2002. Magnetic field orientation and spatial effects on the retention of paramagnetic nanoparticles with magnetite. Separation Science and Technology 37(16):3727–3753.
Shakeri-Zadeh, Ali, Khoee, Sepideh, Shiran, Mohammad-Bagher, Sharifi, Ali Mohammad & Khoei, Samideh . 2010. Synergistic effects of magnetic drug targeting using a newly developed nanocapsule and tumor irradiation by ultrasound on CT26 tumors in BALB/c mice. Journal of Materials Chemistry B 3(9):1879–1887.
Alphandéry, E . 2014. Applications of magnetosomes synthesized by magnetotactic bacteria in medicine. Frontiers in Bioengineering and biotechnology 2:5.
Hola, Katerina, Markova, Zdenka, Zoppellaro, Giorgio, Tucek, Jiri & Zboril, Radek . 2015. Tailored functionalization of iron oxide nanoparticles for MRI, drug delivery, magnetic separation and immobilization of biosubstances. Biotechnology Advances 33(6):1162–1176.
Rehman, Mubashar, Madni, Asadullah, Khan, Waheed Shamraiz, Ihsan, Ayesha, Khan, Muhammad Imran, Mahmood, Muhammad Ahmad, Ashfaq, Muhammad, Bajwa, Sadia Zafar & Shakir, Imran . 2015. Solid and liquid lipid-based binary solid lipid nanoparticles of diacerein: in vitro evaluation of sustained release, simultaneous loading of gold nanoparticles, and potential thermoresponsive behavior. International Journal of Nanomedicine 10:2805.
Chan, Ming-Hsien, Lu, Chih-Ning, Chung, Yi-Lung, Chang, Yu-Chan, Li, Chien-Hsiu, Chen, Chi-Long, Wei, Da-Hua & Hsiao, Michael . 2021. Magnetically guided theranostics: montmorillonite-based iron/platinum nanoparticles for enhancing in situ MRI contrast and hepatocellular carcinoma treatment. Journal of Nanobiotechnology 19:308.
Wang, Chunyan, Ravi, Sowndharya, Garapati, Ujjwala Sree, Das, Mahasweta, Howell, Mark, Mallela, Jaya, Alwarappan, Subbiah, Mohapatra, Shyam S & Mohapatra, Subhra S . 2013. Multifunctional chitosan magnetic-graphene (CMG) nanoparticles: a theranostic platform for tumor-targeted co-delivery of drugs, genes and MRI contrast agents. Journal of Materials Chemistry B 1(35):4396.
Gao, Wei, Kagan, Daniel, Pak, On Shun, Clawson, Corbin, Campuzano, Susana, Chuluun-Erdene, Erdembileg, Shipton, Erik, Fullerton, Eric E, Zhang, Liangfang, Lauga, Eric & Wang, Joseph . 2012. Cargo-Towing Fuel-Free Magnetic Nanoswimmers for Targeted Drug Delivery. Small 8(3):460–467.
Allen, Theresa M & Cullis, Pieter R . 2013. Liposomal drug delivery systems: From concept to clinical applications. Advanced Drug Delivery Reviews 65(1):36–48.
Strijkers, Gustav J, Kluza, Ewelina, Tilborg, Geralda A F Van, Schaft, Daisy W J Van Der, Griffioen, Arjan W, Mulder, Willem J M & Nicolay, Klaas . 2010. Paramagnetic and fluorescent liposomes for target-specific imaging and therapy of tumor angiogenesis. Angiogenesis 13(2):161–173.
Pradhan, P, Giri, J, Rieken, F, Koch, C, Mykhaylyk, O, Döblinger, M, Banerjee, R, Bahadur, D, Plank, C, Edwards, K A, Peng, Z, Fang, E, Wang, C, Lu, X, Wang, G & Tong, Q . 2010. Present and Potential Applications for Magnetic Liposomes in Analysis. Liposomes in Analytical Methodologies. In: Liposomes in Analytical Methodologies. (Vol. 142, pp. 3823-3833) Pan Satnford Publishing
Chen, Wenjie, Cui, Haixin, Zhao, Xiang, Cui, Jinhui, Wang, Yan, Sun, Chaojiao, Cui, Bo & Lei, Feng . 2015. Characterization and Insights Into the Nano Liposomal Magnetic Gene Vector Used for Cell Co-Transfection. Journal of Nanoscience and Nanotechnology 15(8):5530–5536.
Li, Yuhui, Huang, Guoyou, Zhang, Xiaohui, Li, Baoqiang, Chen, Yongmei, Lu, Tingli, Lu, Tian Jian & Xu, Feng . 2013. Magnetic Hydrogels and Their Potential Biomedical Applications. Advanced Functional Materials 23(6):660–672.
Liang, Yuan-Yuan Y, Zhang, Li-Ming M, Jiang, Wei & Li, Wei . 2007. Embedding Magnetic Nanoparticles into Polysaccharide-Based Hydrogels for Magnetically Assisted Bioseparation. ChemPhysChem 8(16):2367–2372.
Beaune, Grégory & Ménager, Christine . 2010. In situ precipitation of magnetic fluid encapsulated in giant liposomes. Journal of Colloid and Interface Science 343(1):396–399.
Shin, M K, Kim, S I, Kim, S J, Park, S Y, Hyun, Y H, Lee, Y, Lee, K E, Han, S.-S, Jang, D.-P & Kim, Y.-B . 2008. Controlled magnetic nanofiber hydrogels by clustering ferritin. Langmuir 24:12107–12111.
Barbucci, Rolando, Giardino, Roberto, Cagna, Milena De, Golini, Lucia & Pasqui, Daniela . 2010. Inter-penetrating hydrogels (IPHs) as a new class of injectable polysaccharide hydrogels with thixotropic nature and interesting mechanical and biological properties. Soft Matter 6(15):3524.
Campbell, Scott, Maitland, Danielle & Hoare, Todd . 2015. Enhanced Pulsatile Drug Release from Injectable Magnetic Hydrogels with Embedded Thermosensitive Microgels. ACS Macro Letters 4(3):312–316.
Deidier, A . 1725. Deux dissertations medecinales et chirurgicales, l'une sur la maladie venerienne, l'autre sur la nature & la curation des tumeurs. chez Charles Maurice.
Busch, W . 1867. Aus der Sitzung der medicinischen Section vom 13. Berl Klin Wochenschr 5(5):137.
Yerushalmi, A, Brenner, H & Yerushalmi, A . 1976. Treatment of a solid tumor by local simultaneous hyperthermia and ionizing radiation: Dependence on temperature and dose. European Journal of Cancer (1965) 12(10):807–813.
Umair, Muhammad, Javed, Ibrahim, Rehman, Mubashar, Madni, Asadullah, Javeed, Aqeel, Ghafoor, Aamir & Ashraf, Muhammad . 2016. Nanotoxicity of Inert Materials: The Case of Gold, Silver and Iron. Journal of Pharmacy & Pharmaceutical Sciences 19(2):161.
Bazylinski, Dennis A & Frankel, Richard B . 2004. Magnetosome formation in prokaryotes. Nature Reviews Microbiology 2(3):217–230.
Ivanova, A V, Nikitin, A A, Gabashvily, A N, Vishnevskiy, D A & Abakumov, M A . 2021. Synthesis and intensive analysis of antibody labeled single core magnetic nanoparticles for targeted delivery to the cell membrane. Journal of Magnetism and Magnetic Materials 521:167487.
Ito, Akira, Tanaka, Kour, Honda, Hiroyuki, Abe, Shigeru, Yamaguchi, Hideyo & Kobayashi, Takeshi . 2003. Complete regression of mouse mammary carcinoma with a size greater than 15 mm by frequent repeated hyperthermia using magnetite nanoparticles. Journal of Bioscience and Bioengineering 96(4):364–369.
Hegyi, G, Szigeti, G P & Szász, A . 2013. Hyperthermia versus oncothermia: cellular effects in complementary cancer therapy. Evidence-Based Complementary and Alternative Medicine 12:672873.
Roti, J L Roti . 2008. Cellular responses to hyperthermia (40–46°C): Cell killing and molecular events. International Journal of Hyperthermia 24(1):3–15.
Lutgens, Ludy C H W, Koper, Peter C M, Jobsen, Jan J, Steen-Banasik, Elzbieta M Van Der, Creutzberg, Carien L, Berg, Hetty A Van Den, Ottevanger, Petronella B, Rhoon, Gerard C Van, Doorn, Helena C Van, Houben, Ruud & Zee, Jacoba Van Der . 2016. Radiation therapy combined with hyperthermia versus cisplatin for locally advanced cervical cancer: Results of the randomized RADCHOC trial. Radiotherapy and Oncology 120(3):378–382.
Sato, Itaru, Umemura, Masanari, Mitsudo, Kenji, Fukumura, Hidenobu, Kim, Jeong-Hwan, Hoshino, Yujiro, Nakashima, Hideyuki, Kioi, Mitomu, Nakakaji, Rina, Sato, Motohiko, Fujita, Takayuki, Yokoyama, Utako, Okumura, Satoshi, Oshiro, Hisashi, Eguchi, Haruki, Tohnai, Iwai & Ishikawa, Yoshihiro . 2016. Simultaneous hyperthermia-chemotherapy with controlled drug delivery using single-drug nanoparticles. Scientific Reports 6(1):6.
Diederich, C J . 2005. Thermal ablation and high-temperature thermal therapy: Overview of technology and clinical implementation. International Journal of Hyperthermia 21(8):745–753.
Imai, T, Kikumori, T, Akiyama, M, Yokoyama, K, Nishida, Y & Fujimoto, Y . 2011. A phase I study of hyperthermia using magnetite cationic liposome and alternating magnetic field for various refractory malignancies. In: In 28th annual meeting of Japan society for thermal medicine. 3–6.
Khalid, Q . 2021. Negative Effects of Lockdown: Revisiting Obesity And Cancer Risk. Negative Effects of Lockdown: Revisiting Obesity And Cancer Risks. HealthCreeds. https://healthcreeds.com/negative-effects-of-lockdown-revisiting-obesity-and-cancer-risks/
Munir, Tariq, Mahmood, Arslan, Fakhar-E-Alam, Muhammad, Imran, Muhammad, Sohail, Amjad, Amin, Nasir, Latif, Sadia, Rasool, Hafiz Ghullam, Shafiq, Fahad, Ali, Haider & Mahmood, Khalid . 2019. Treatment of breast cancer with capped magnetic-NPs induced hyperthermia therapy. Journal of Molecular Structure 1196:88–95.
Beola, Lilianne, Grazú, Valeria, Fernández-Afonso, Yilian, Fratila, Raluca M, Heras, Marcelo De Las, Fuente, Jesús M De La, Gutiérrez, Lucía & Asín, Laura . 2021. Critical Parameters to Improve Pancreatic Cancer Treatment Using Magnetic Hyperthermia: Field Conditions, Immune Response, and Particle Biodistribution. ACS Applied Materials & Interfaces 13(11):12982–12996.
Mahmoudi, Keon, Bouras, Alexandros, Bozec, Dominique, Ivkov, Robert & Hadjipanayis, Constantinos . 2018. Magnetic hyperthermia therapy for the treatment of glioblastoma: a review of the therapy’s history, efficacy and application in humans. International Journal of Hyperthermia 34(8):1316–1328.
Dodd, K, Nance, S, Quezada, M, Janke, L, Morrison, J B, Williams, R T & Beere, H M . 2015. Tumor-derived inducible heat-shock protein 70 (HSP70) is an essential component of anti-tumor immunity. Oncogene 34(10):1312–1322.
Udono, H & Srivastava, P K . 1994. Comparison of tumor-specific immunogenicities of stress-induced proteins gp96, hsp90, and hsp70. The Journal of Immunology 152(11):5398–5403.
Shevtsov, Maxim & Multhoff, Gabriele . 2016. Heat Shock Protein–Peptide and HSP-Based Immunotherapies for the Treatment of Cancer. Frontiers in Immunology 7:7.
Raaijmakers, E, Paulides, M, Mestrom, R, Stakhursky, V L, Arabe, O, Cheng, K.-S, Macfall, J, Maccarini, P, Craciunescu, O, Dewhirst, M, Stauffer, P, Das, S K, Senneville, B D De, Moonen, C & Ries, M . 2009. Real-time MRI-guided hyperthermia treatment using a fast adaptive algorithm. Therapeutic Ultrasound 54:43–63.
Gao, Lizeng, Zhuang, Jie, Nie, Leng, Zhang, Jinbin, Zhang, Yu, Gu, Ning, Wang, Taihong, Feng, Jing, Yang, Dongling, Perrett, Sarah & Yan, Xiyun . 2007. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nature Nanotechnology 2(9):577–583.
Liu, B, Sun, Z, Huang, P.-J J, Liu, J, Wang, H, Li, S, Si, Y, Zhang, N, Sun, Z, Wu, H & Lin, Y . 2014. Platinum nanocatalysts loaded on graphene oxide-dispersed carbon nanotubes with greatly enhanced peroxidase-like catalysis and electrocatalysis activities. Journal of the American Chemical Society 2015(3):8107–8116.
Wei, H & Wang, E . 2008. Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Analytical chemistry 80(6):2250–2254.
Qian, Jing, Yang, Xingwang, Jiang, Ling, Zhu, Chendan, Mao, Hanping & Wang, Kun . 2014. Facile preparation of Fe3O4 nanospheres/reduced graphene oxide nanocomposites with high peroxidase-like activity for sensitive and selective colorimetric detection of acetylcholine. Sensors and Actuators B: Chemical 201:160–166.
Kaushik, Ajeet, Khan, Raju, Solanki, Pratima R, Pandey, Pratibha, Alam, Javed, Ahmad, Sharif & Malhotra, B D . 2008. Iron oxide nanoparticles–chitosan composite based glucose biosensor. Biosensors and Bioelectronics 24(4):676–683.
Wang, Yan, Zhang, Xiuhua, Chen, Yang, Xu, Hui, Tan, Yumei & Wang, Shengfu . Detection of Dopamine Based on Tyrosinase-Fe3O4 Nanoparticles-chitosan Nanocomposite Biosensor. American Journal of Biomedical Sciences 2010(3):209–216.
Bi, Changfen, Zhao, Yingran, Shen, Lijin, Zhang, Kai, He, Xiwen, Chen, Langxing & Zhang, Yukui . 2015. Click Synthesis of Hydrophilic Maltose-Functionalized Iron Oxide Magnetic Nanoparticles Based on Dopamine Anchors for Highly Selective Enrichment of Glycopeptides. ACS Applied Materials & Interfaces 7(44):24670–24678.
Levine, G N, Gomes, A S, Arai, A E, Bluemke, D A, Flamm, S D, Kanal, E, Manning, W J, Martin, E T, Smith, J M & Wilke, N . 2007. Safety of Magnetic Resonance Imaging in Patients With Cardiovascular Devices An American Heart Association Scientific Statement From the Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Council on Cardiovascular Radiology and Intervention: Endorsed by the American College of Cardiology Foundation, the North American Society for Cardiac Imaging, and the Society for Cardiovascular Magnetic Resonance. Circulation 116(24):2878–2891.
Chaljub, Gregory, Kramer, Larry A, Johnson, Raleigh F, Johnson, Raleigh F, Singh, Harbans F & Crow, Wayne N . 2001. Projectile Cylinder Accidents Resulting from the Presence of Ferromagnetic Nitrous Oxide or Oxygen Tanks in the MR Suite. American Journal of Roentgenology 177(1):27–30.
Mild, K H, Mattsso, M.-O, Hardell, L, Bowman, J D & Kundi, M . 2005. Occupational carcinogens: ELF MFs. Environmental health perspectives 113(11):726.
Cho, Yoon Hee, Jeon, Hye Kyoung & Chung, Hai Won . 2007. Effects of Extremely Low-Frequency Electromagnetic Fields on Delayed Chromosomal Instability Induced by Bleomycin in Normal Human Fibroblast Cells. Journal of Toxicology and Environmental Health, Part A 70(15-16):1252–1258.
Ghodbane, Soumaya, Lahbib, Aida, Sakly, Mohsen & Abdelmelek, Hafedh . 2013. Bioeffects of Static Magnetic Fields: Oxidative Stress, Genotoxic Effects, and Cancer Studies. BioMed Research International 2013:1–12.
Schwenzer, Nina F, Bantleon, Rüdiger, Maurer, Brigitte, Kehlbach, Rainer, Schraml, Christina, Claussen, Claus D & Rodegerdts, Enno . 2007. Detection of DNA double-strand breaks using γh2AX after MRI exposure at 3 Tesla: An in vitro study. Journal of Magnetic Resonance Imaging 26(5):1308–1314.
Österberg, K, Persson, R, Karlson, B, Eek, F C & Ørbæk, P . 2007. Personality, mental distress, and subjective health complaints among persons with environmental annoyance. Human & Experimental Toxicology 26(3):231–241.
Houpt, Thomas A, Cassell, Jennifer A, Riccardi, Christina, Denbleyker, Megan D, Hood, Alison & Smith, James C . 2007. Rats avoid high magnetic fields: Dependence on an intact vestibular system. Physiology & Behavior 92(4):741–747.
Mcfadden, J . 2013. The CEMI Field Theory Closing the Loop. Journal of Consciousness Studies 20(1-2):153–168.
Alabdulgader, A A, Mccraty, R & Zayas, M A . 2012. Coherence: A Novel Nonpharmacological Modality for Lowering Blood Pressure in Hypertensive Patients. Global Advances in Health and Medicine 1(2):56–64.
Keywords: MRI,nanoparticles,liposomes,hydrogels,hyperthermia,thermoresponsive
Materials Innovations (MI) is an interdisciplinary journal devoted to significant experimental and theoretical findings on the synthesis, structure, charachterization, processing and applications of materials. Materials Innovations is dedicated to publishing peer reviewed novel, cutting edge reports of broad interest to the materials science community.