
Full Html
Vol 2 Issue 5
High Performance of SDC Composite Electrolyte Using Natural Gas as a Fuel for Low Temperature SOFC
Pages: 132-138
Doi: 10.54738/MI.2022.2501
Doi URL: http://doi.org/10.54738/MI.2022.2501
Nazia Yasmin 1 , Muhammad Safdar ?? 2 , Aisha Iftikhar 1 , Mukhtar Ahmad 3 , Misbah Mirza ?? 1
1 The Women University Multan, Pakistan
2 Department of basic sciences & humanities, Department of Physics , Khawaja Fareed University of Engineering & Information, Lahore, Pakistan
3 Department of Physics, COMSATS Institute of Information Technology, Lahore, 54000, Pakistan
In this work, nanocomposite of Lithium and Samarium doped Ceria (Li-SDC) is synthesized by means of polyol process to obtain dense electrolyte material for low temperature Solid Oxide Fuel Cell (SOFCs) without any chelating agent. The crystalline structure of nanocomposite material is examined by X-ray diffraction (XRD). The fuel cell performance is obtained at temperature range 500-550 ?C. The conductivity of material was measured by 4-probe method. The prepared material morphology and microstructure analysis was studied by scanning electron microscope (SEM) images. The best results are obtained with sample which was prepared by polyol process gives 0.016 S/cm conductivity and it shows maximum power density of 0.2 W/cm2 at 500 ?C and 0.3 W/cm2 at 550 ?C respectively using natural gas as fuel. These results prevailed that the prepared electrolyte using polyol is best for low temperature SOFC. It is also noticed that as electrolyte ionic conductivity increases the performance of cell is also enhanced.
Keywords
Nanocomposite, LiSDC, Conductivity, SOFC, Electrolyte
At present, electrical power is considered as important part of our lives. Sustainable energy, steadfast and environmental friendly power resources are the backbone to enhance the standard of current lifestyle. The reduction in the fossil fuels attracts the attention of researchers towards alternative energy resources. Fuel cells, for example, have been around for more than 1.5 centuries and provide an endless source of energy that is both environmental friendly and always available. Fuel cells have become increasingly popular over the last decade, with the goal of laying the ground work for future innovation, as they provide high efficiencies and are highly adaptable in power applications.The bulk of energy organizations are focusing on this invention, and there is currently a thriving commercial company exchanging fuel cells. Power corporations’ residence relies upon on the two variables, cost and the long closing1, 2, 3.
Among all fuel cells kinds, SOFCs are most tempting because of its higher performance and low pollution. Numerous researchers concentrated on SOFCs toattain the exceptional performance by thecell. They mixed a variety of combos of SOFCs, in order to achieve extremely high energy densities and exceptional conductivity at low temperature. By improving reaction at electrodes and enhancing ionic conductivity of electrolyte many researchers are trying to lower the working temperature of SOFCs 4, 5. This can be obtained by using such fuels (like CH4, H2, H2S, C3H8 etc.) that not only improve life time but also reduced the cost of cell. Gadolinium doped Ceria (GDC), Yttria stabilized Zirconia (YSZ), Scandia stabilized Zirconia (ScSZ), Yttria doped-Ceria (YDC), Samarium doped Ceria (SDC), and many other ceria composites are utilized to enhance electrolytes ionic conductivity. At low temperatures i.e. less than 700 ?C as compared to zirconia based electrolytes the ionic conductivity of electrolytes which based on ceria are better. Ionic conductivity depends on composition and crystalline quality. However, better ionic conductivity can be got through grain boundaries and crystalline size 6, 7. Both ionic and electronic conduction is established by pure CeO2 fluorite type structure but on the other hand has poor oxygen ion conduction. With the help of rare earth elements like Gd+3 and Sm+3 the oxygen ion conductivity might be improved. These low valence dopant cations not only enhance oxygen ion conduction but also increase ionic and electronic conductivities 6, 8.
At low temperatures ranging from 450-700 ?C doped electrolytes of ceria base demonstrate better efficiency but due to mixed conduction their efficiencies remain lower. As electrolytes ionic conductivity increased the efficiency of cell may also be increased. Different researchers used different approaches to enhance the ionic conduction. Alkaline salts in doped ceria create a new phase called the second phase with matrix phase ceria doped. For SOFCs natural gas is getting a valuable attention. For generation of energy hydrocarbon direct use results into deposition of higher temperature 6, 7, 8.
In present work Lithium ceria composite as electrolyte are synthesized while its structure, cell performance and thermal stability are investigated by using natural gas as fuel.
Lithium Samarium doped Ceria (Li-SDC) as electrolyte for SOFC prepared by using polyol process.
With the use of a measuring balance, I measured 13.02 g of Ce(NO3)2.6H2O, 1.229 g of Li(CO3), and 4.44 g of Sm(NO3 )3.6H2O. As a reducing agent, 100mL tetra-ethylene glycol dimethyl ether was placed in a beaker and heated and stirred continuously using a magnetic stirrer. The temperature of the magnetic stirrer was set at 200 ?C. After 5 minutes, add Ce(NO3) to the tetra ethylene glycol dimethyl ether and wait 2–5 minutes. Then, in the aforesaid solution, add samarium nitrate and stirred for 30 minutes while heating. After 30 minutes of samarium nitrate addition, had added lithium carbonate to the reducing reagent and stir constantly for another 30 minutes while heating on the magnetic stirrer at 200 ?C. The solution changed the color during this procedure in the following pattern
White → yellow → dark brown
Solution is cooled down at room temperature. Centrifugation is utilized to separate the solid particles from the solution. The de-ionized water or ethanol was used in filtration process to wash the remained solid solution in centrifugation. When filtration process was completed the remained solids were put in the pettry dish and put the pettry dish in` oven at 200 ?C for drying the remained solids solution. Then crushed this attained dry powder and put it in crucible and covered the crucible with lid and kept in furnace for calcinations in stepwise one hour at each temperature 300 ?C, 500 ?C, 750 ?C, then placed used 4 hour at 850 ?C respectively. The final obtained powder after calcination; it was grounded for further characterization. Crushed the calcinated specimen and made two pallets via applying pressure on it. Figure 1 illustrates the experimental detail.
• One was complete fuel cell with anode, electrolyte and cathode. The composite anode Ni/Zn oxide material and cathode was BSCF (barium strontium cobalt iron oxide) conventional material were used for cell preparation and testing.
• Other was pure electrolyte pellet (Li-SDC) for conductivity measurements
Fuel cell pellet has 0.64 cm2 active area.
Pellet thickness is 1mm and diameter 13 mm.
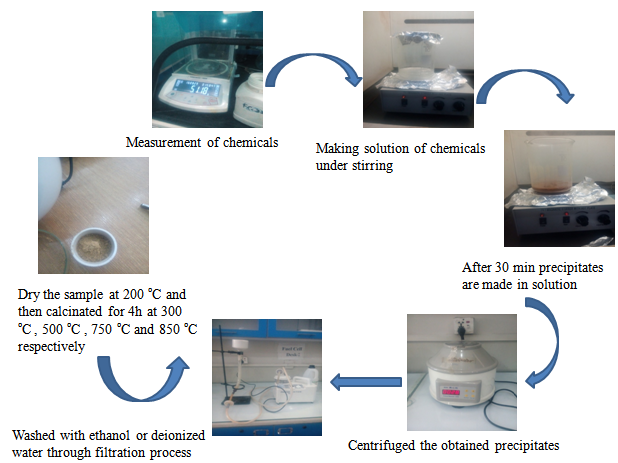
Figure 1: Schematic illustration of experimental details.
Figure 2(a) reveals XRD pattern of Li-Samarium doped Ceria (Li-SDC). All peaks in the XRD pattern reveals that there is no evidence of SmO due to their proper doping in ceria. The crystallite sizes (D) were determinedthrough the Scherrer’s formula given below:
D = K λ/ βcosθ
Where θ is the angle between X-ray beam and sample.
λ → X-ray wavelength
β → fullwidth at half maxima (FWHM)
D → crystallite size
Nano particle assemblies allow three dimensional confinements that lead synthesis of polygonal nano crystal of (Li-SDC) with size 200-500 nm. In this XRD pattern, high temperature treatment during fabrication caused high diffraction intensities 9, and better crystalline are formed at higher sintering temperature 10.
Figure 2(b) reveals SEM analysis of prepared samples. SEM showed high density, with well-defined grains shape and homogeneous surface. To evade stresses in the specimen the process sintering temperatures was determined warily and clogged the heating when the grains started to demonstrate slip bands. At 800 ?C dense microstructures has been showed by Li doped SDC. By increasing sintering temperatures and Li doping may reinforce grains growth. The observed averagegrain size was found 200-500 nm. Because of nano particles the conductivity of our composite is high. The size of grain boundaries of these nanoparticles are large which is also favorable for increased conductivity of composites11 as by this contact resistance reduces which provide a path way for ions to pass through interfaces of particles more easily 12, 13.
The Li doped SDC showed dense microstructures at 800 ?C because presence of Li2O encourages densification. At 247.4 ?C lithium nitrate starts melting while at 485 ?C it starts decomposing 14. Lithium oxide is formed and also possibly on the surface of Li-SDC grain, liquid of Ce-Sm-Li-O formed during sintering 15. At the grain surface of SDC it seems that Li2O adsorbed and formed thick layer around SDC. It may make sense that during sintering it will help SDC to transport and rearrange by enhancing the diffusion rate 16, and then densification was increased and hence required sintering energy was reduced. Li2O lowered the sintering temperature of SDC by changing core structure of grain boundary. Therefore, at grain boundary of SDC more Li2O will stay 17.

Figure 2: SEM images of Lithium and Samarium doped Ceria (Li-SDC).
The polarization curve shows the fuel cell's voltage output for a particular current density loading.
Fuel cell polarization curve has three main regions:
• The activation polarization causes the cell potential to decline at low power densities.
• Because of ohmic losses, the cell potential drops linearly with current at moderate current densities.
• Due to more distinct concentration polarization, the cell potential drop deviates from the linear relationship with current density at high current densities.
Schematic measuring setup of IV/IP curve is shown in Figure 3. When a fuel cell's current (load) is changed, the fuel cell's heat and water balance shifts, and it can take some time to find a new equilibrium point. During testing, the fuel cell should be given a set amount of time to attain its new equilibrium. Whether the fuel cell load has been increased or decreased affects the formation of an equilibrium phase. The load can be designed to rise or decrease by a specified step-size, or the load can be randomly selected to collect relevant test data from the fuel cell in a various ways. The most common way is to gradually raise the load. Multiple current or voltage points can be used to collect data. Beginning with open-circuit voltage is a common way for taking measurements. Reliable voltage/current curves necessitate a stable environment in which pressure, humidity, flow rates and temperature remain constant throughout the test. The voltage/current characteristics may alter if the conditions change. In our case polarization curve is shown in Figure 4(a).
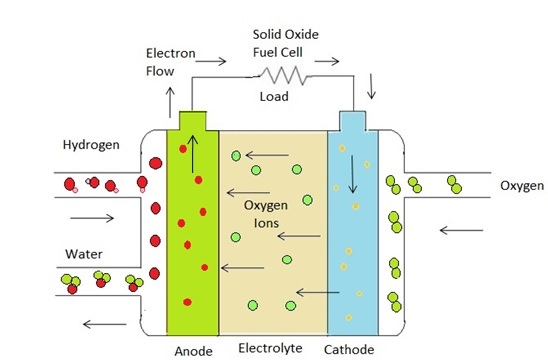
Figure 3: Schematic measuring setup of IV/IP curves.
Fuel cell performance with the synthesized electrolyte of Li-SDC has been inspected in 500 to 550 ?C temperature range. During this process natural gas is utilized as fuel in this cell and the supply rate is 100 mlmin-1 at 1 atm pressure at anode side and air is provided towards cathode side by using air pump. Figure 4(a) shows IV/IP curves provided maximum power density of 0.2 W/cm2 at 500 ?C and 0.3 W/cm2 at 550 ?C respectively 18 . The fuel cell performance shows that the prepared electrolyte has very good compatibility with the electrodes used in the cell. The IV curve showed that there is very small polarization losses at low temperature as compare to the high temperature reported work. The maximum obtained open circuit voltage (OCV) was 0.9 V at 550 ?C. It is further noticed that the small drop of OCV and power density is due to the dense composite electrolyte which was obtained due to this new polyol preparation method.

Figure 4: (a) The fuel cell performance Lithium and Samarium doped Ceria (Li-SDC) With Natural Gas as fuel. (b) Conductivity of prepared electrolyte (Li-SDC).
The Li-SDC ionic conductivity was tested with the help of four probe dc technique of with 13 mm pellet which was pressed under pressure of 300 MP with hydraulic press. For good conductivitysilver paste was utilized to paintthe synthesized pellets. The obtained results were plotted through origin pro 8.0 software. The conductivity is calculated by given formula:
σ = L/RA
Where, σ is ionic conductivity,A is active surface area of pallet, R is internal resistance and L is thickness of pallet. Plotted Arrhenius graph showed conductivity of 0.01 S/cm19 as shown in Figure 4(b). As reported by other researchers, due to low Ohmic losses, the performance of SDC based SOFCs is superior as compared to others 20, 21, 22 . According to GuO and Waser 23, the decrease of Schottky barrier height (?Φ) is due to Li+ enrichment, while the mechanism of lowering Schottky barrier height in SOFC is shown in Figure 5. Thus in space charge layers, it reduces the depletion of oxygen vacancies. Due to presence of Li2O with SDC it results in enhancement of conductivity of grain boundaries 17.
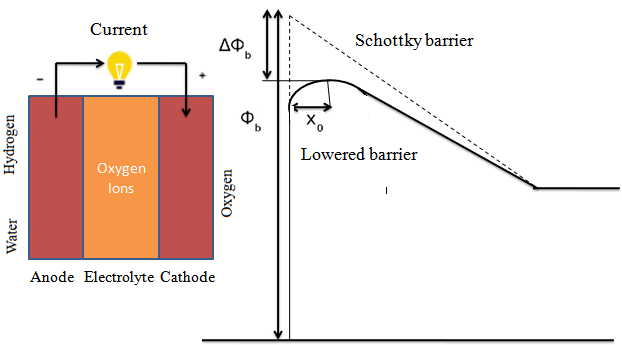
Figure 5: Mechanism of lowering Schottky barrier height in SOFC.
Nanocomposites Lithium Samarium doped Ceria (Li-SDC) was synthesized using a polyol and developed as electrolyte for SOFCs. In present work IV/IP curves give maximum power density of 0.3 W/cm2 at 550 ?C. The maximum conductivity of 0.016 S/cm was achieved which is sufficient for any good fuel cell. The improved performance of fuel cell, conductivity and stability of the cell revealed that the prepared electrolyte is very dense and up to the mark for low temperature SOFC (solid oxide fuel cell). Hence, Li-SDC can be used as electrolyte for low temperature SOFCs by using natural gas as a fuel. Also, during sintering, at boundaries Lithium stays as liquid, which helps to enhance the conductivity of grain boundaries and also activate the surface of SDC at low temperature. In grain boundary of space charge layer, the depletion of oxygen vacancies depleted which results in enrichment of concentration of oxygen vacancy in grain boundary which leads to increase in conductivity of SDC.
Patnaik, P . 1995. Dean’s Analytical Chemistry Handbook. The McGraw-Hill Companies, Inc
Klein, L C & Garvey, G J . 1980. Kinetics of the sol/gel transition. Journal of Non-Crystalline Solids (1) 90392.
Brinker, C J, Keefer, K D, Schaefer, D W & Ashley, C S . 1982. Sol-gel transition in simple silicates. Journal of Non-Crystalline Solids 48(1):47–64.
Muhammad, A, Muchtar, S A, Muhammad, N & Sulong, A B . 2011. A Review on Preparation of SDC-Carbonate as Composite Electrolyte Material for Intermediate Temperature Solid Oxide Fuel Cells (IT-SOFC)First Conference on Clean Energy and Technology CET 394–399.
Gao, Zhan, Huang, Jianbing, Mao, Zongqiang, Wang, Cheng & Liu, Zhixiang . 2010. Preparation and characterization of nanocrystalline Ce0.8Sm0.2O1.9 for low temperature solid oxide fuel cells based on composite electrolyte. International Journal of Hydrogen Energy 35(2):731–737.
Hui, Shiqiang (rob), Roller, Justin, Yick, Sing, Zhang, Xinge, Decès-Petit, Cyrille, Xie, Yongsong, Maric, Radenka & Ghosh, Dave . 2007. A brief review of the ionic conductivity enhancement for selected oxide electrolytes. Journal of Power Sources 172(2):493–502.
Shemilt, J E & Williams, H M . 1999. Effects of composition and processing method on the low temperature conductivity of samaria-doped ceria electrolytes. Journal of Materials Science Letters 18:1735–1737.
Menzler, Norbert H, Tietz, Frank, Uhlenbruck, Sven, Buchkremer, Hans Peter & Stöver, Detlev . 2010. Materials and manufacturing technologies for solid oxide fuel cells. Journal of Materials Science45(12):3109–3135.
Li, , Yan, Zi F, Lu, Gao Q & Zhu, Zhong H . 2006. Synthesis and Structure Characterization of Chromium Oxide Prepared by Solid Thermal Decomposition Reaction. The Journal of Physical Chemistry B110(1):178–183.
Changshi, L . 2011. Prediction of the Magneto-Resistance of La0.67Ca0.33MnO3 and La0.8Sr0.2MnO3 via Temperature and a Magnetic Field. Journal of Chemical & Engineering Data 56(1):2–8.
Mohammed, M I, Elbadawi, A A & Abuellhassan, H H . 2013. Temperature and Grain Size Effect on the Electrical Conductivity of La0.67Ca0.33MnO3. Journal of Applied and Industrial Sciences 1(3):12–22.
Wang, Xiaodi, Ma, Ying, Li, Shanghua, Zhu, Bin & Muhammed, Mamoun . 2012. SDC/Na2CO3 nanocomposite: New freeze drying based synthesis and application as electrolyte in low-temperature solid oxide fuel cells. International Journal of Hydrogen Energy 37(24):19380–19387.
Wang, Xiaodi, Ma, Ying, Raza, Rizwan, Muhammed, Mamoun & Zhu, Bin . 2008. Novel core–shell SDC/amorphous Na2CO3 nanocomposite electrolyte for low-temperature SOFCs. Electrochemistry Communications 10(10):1617–1620.
Ruiz, Maria Lucia, Lick, Ileana Daniela, Ponzi, Marta Isabel, Castellón, Enrique Rodríguez, Jiménez-López, Antonio & Ponzi, Esther Natalia . 2010. Thermal decomposition of supported lithium nitrate catalysts. Thermochimica Acta 499(1-2):21–26.
Nicholas, J D & Dejonghe, L . 2007. Prediction and evaluation of sintering aids for Cerium Gadolinium Oxide. Solid State Ionics 178(19-20):1187–1194.
Jud, Eva, Huwiler, Christoph B & Gauckler, Ludwig J . 2005. Sintering Analysis of Undoped and Cobalt Oxide Doped Ceria Solid Solutions. Journal of the American Ceramic Society 88(11):3013–3019.
Han, M, Liu, Z, Zhou, S & Yu, L . 2011. Influence of Lithium Oxide Addition on the Sintering Behavior and Electrical Conductivity of Gadolinia Doped Ceria. Journal of Materials Science & Technology27(5):60091–60092.
Fan, Liangdong, Zhu, Bin, Chen, Mingming, Wang, Chengyang, Raza, Rizwan, Qin, Haiying, Wang, Xuetao, Wang, Xiaodi & Ma, Ying . 2012. High performance transition metal oxide composite cathode for low temperature solid oxide fuel cells. Journal of Power Sources 203:65–71.
Khan, M Ajmal, Raza, Rizwan, Lima, Raquel. B, Chaudhry, M Asharf, Ahmed, E & Abbas, Ghazanfar . 2013. Comparative study of the nano-composite electrolytes based on samaria-doped ceria for low temperature solid oxide fuel cells (LT-SOFCs) International Journal of Hydrogen Energy 38(36):16524–16531.
Aguiar, P, Adjiman, C S & Brandon, N P . 2004. Anode-supported intermediate temperature direct internal reforming solid oxide fuel cell. I: model-based steady-state performance. Journal of Power Sources 138(1-2):120–136.
Dumaisnil, K, Fasquelle, D, Mascot, M, Rolle, A, Roussel, P, Minaud, S, Duponchel, B, Vannier, R-N N & Carru, J-C C . 2014. Synthesis and characterization of La0.6Sr0.4Co0.8Fe0.2O3 films for solid oxide fuel cell cathodes. Thin Solid Films 553(28):89–92.
Saebea, Dang, Authayanun, Suthida, Patcharavorachot, Yaneeporn, Chatrattanawet, Narissara & Arpornwichanop, Amornchai . 2018. Electrochemical performance assessment of low-temperature solid oxide fuel cell with YSZ-based and SDC-based electrolytes. International Journal of Hydrogen Energy 43(2):921–931.
Guo, X & Waser, R . 2006. Electrical properties of the grain boundaries of oxygen ion conductors: Acceptor-doped zirconia and ceria. Progress in Materials Science 51(2):151–210.
Keywords: Nanocomposite,Li-SDC,Conductivity,SOFC,Electrolyte
Materials Innovations (MI) is an interdisciplinary journal devoted to significant experimental and theoretical findings on the synthesis, structure, charachterization, processing and applications of materials. Materials Innovations is dedicated to publishing peer reviewed novel, cutting edge reports of broad interest to the materials science community.