
Full Html
Vol 2 Issue 8 (Special Issue)
A Mini Review on Ni-rich Layered Oxide Cathode Materials
Pages: 197-202
Doi: 10.54738/MI.2022.2802
Doi URL: http://doi.org/10.54738/MI.2022.2802
Sidra Jamil ?? 1 , Muhammad Fasehullah 2
1 Department of Physics, NED University of Engineering and Technology, Karachi, 75270, Pakistan
2 State key laboratory of Power Transmission Equipment & System Security and New Technology,, Chongqing University, Chongqing, 400044, China
The global energy demand and increasing global warming are threats to the planet; therefore, replacing internal combustion engine vehicles to electric vehicles is prerequisite. Rechargeable batteries are key component to operate electric vehicles, among which lithium-ion batteries are governing the industry. After the successful discovery of Goodenough’s LiCoO2, layered oxides are emerging and are considered to be the next-generation cathodes. Ni-rich layered oxides are the new-generation cathode material owing to high specific capacity, high energy density, high operating voltage and low cost. Substitution of Co, Mn and Al is advantageous to compensate for the loss of the storage capacity and structural degradation. This review aims to discuss the characteristics, structure and preparation methods for nickel-rich layered oxide cathode material.
Keywords
Layered oxide, Cathode materials, Nickel rich, Lithium ion batteries
The worldwide concerns related to global warming and excessive greenhouse gas emissions from burning fossil fuels in internal combustion engine (ICE) vehicles have taken researchers' interest. Meanwhile, the energy demand is also increasing with the rapid growth of the population and the world economy. Therefore, it now becomes a challenge for researchers to improve existing technologies, explore new energy resources, and develop a clean, green, and sustainable environment to fulfill the world's energy needs1-4. Renewable energy sources such as solar energy and wind energy are the focus of energy development for a clean and green environment owing to their advantages over traditional fossil fuels. However, these energy sources are not continuous and mainly dependent on government subsidies. The purchasing cost of a solar PV module has increased by 50 times since 1980 and ten times more in 2005. Similarly, wind technology also requires considerable support and grants from the government, challenging to maintain in a long-term, economically competitive environment5. For a clean, green and sustainable environment, the ICE vehicles should be replaced with electric vehicles (EVs) for which rechargeable batteries are the most indispensable part for the operation of electric vehicles and other energy storage systems6-8. The cost of an EV battery pack is projected to reach 125 US$ kW h–1 in 2022, which is beneficial for the development of electromobility9.
In 1991, lithium-ion batteries (LIBs) with enhanced gravimetric and volumetric energy density was commercialized by Sony for its Walkman10, 11. Subsequently, electric vehicles were fully? equipped with LIBs in 200012. Further, in 2015, a new series of electric car models (Tesla Model S and X, Nissan LEAF, Chevy Volt, Ford C-Max Energi, Toyota Prius Prime, Volkswagen e-Golf, BMW i3, etc.) were introduced, which gained worldwide popularity13. In 2017, the estimated number of EVs reached 695,000 worldwide, with a remarkably increased sale of 50% from past years. China maintains the world's largest automobile industry, with about 50% of EVs sales, which is expected to grow by 2022, as illustrated in Figure 114.
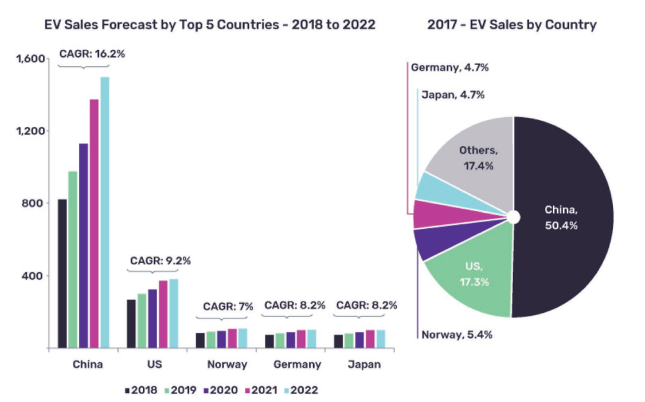
Figure 1: EVs sales for the top five countries in the world14
The journey begins with developing a TiS2 cathode with lithium anode by Whittingham in 197015. LiCoO2 (LCO), discovered in the 1980s by Goodenough, was the first commercialized layered oxide cathode material possessing a high theoretical and volumetric capacity16, 17. Besides, Spinel LiMn2O4 (LMO) becomes another commercialized cathode due to the inexpensive manganese; though, it suffers from severe challenges18. LiFePO4 (LFP) with olivine structure was introduced in 1996 by the University of Texas, owning good chemical and thermal stability19, 20. Lithium titanate also gained attention in electric power trains and smart grids21. For commercialization, the cathode chemistries should be integrated with transition metals (Ni, Co, Mn, or Al) to prepare LiNiCoMnO2 or LiNiCoAlO2 due to cost-effectiveness. Cathode chemistry primarily determine the capacity of LIBs; therefore, enhancing its energy density, cycling stability, thermal stability, and cost-effectiveness is a challenge for commercialization22. According to their chemistries and structures, cathode materials are classified into the following categories23:
1) Layered transition metal oxide (LiMO2) (M=transition metal) cathode material including LiCoO2 ? LiNiO2, LiMnO2, and binary or ternary material LiNixCoyMzO2 (1-x-y-z), (where M is Mn, Al, Mg, and other metals).
2) Spinel transition metal oxides such as lithium manganese-based material LiMn2O4 and LiNi0.5Mn1.5O4.
3) Olivine polyanionic compounds, including transition metal phosphate (LiFePO4) silicate, borate, and pyrophosphate.
4) Other types of cathode material, such as vanadium-based materials and transition metal fluorides.
Recently, the researchers achieved 200-250 Wh kg-1 of specific energy in EV batteries; however, 500 Wh g-1 with a total mileage of 150,000 miles and a driving range of 300 miles per single charge is required to achieve within next ten years8, 12, 24. In a characteristic battery pack, 40% of the performance is dedicated to the cathode material; therefore, the active material's chemistry, structure, and electrochemical performance should be keenly optimized. The charge/ discharge voltage for a commercial lithium battery is typically in the range of 3.4-4.1 V (vs Li+), and the specific discharge capacity is around 200 mAh g-1. To improve the performance of LIBs, the positive active material should have a high specific capacity, high energy and volumetric density, high operating voltage, superior thermal stability, high tap density, low cost, and eco-friendliness25, 26. To achieve high performance from the cathode chemistries along with low cost, the cathode material should have the following characteristics27:
1) The structural stability is high. During the charge/discharge process, the lattice expansion and shrinkage are less convenient to insert/extract more Li+ ions.
2) The material is an excellent ionic and electronic conductor with less impedance at the cathode-electrolyte interface.
3) Redox potential is high and stable during the charge/discharge process.
4) Superior thermal stability.
5) They were synthesized using abundant raw materials with low cost and environmental friendliness.
6) The synthesis process should be facile and have a good yield for large scale production.
Over the last decade, Nickel-based layered oxide materials, such as LiNixCoyMnzO2 and LiNixCoyAlzO2 (x + y + z = 1), were considered the most suitable cathodes for LIBs due to their high specific capacity, high energy density, high operating voltage range, and low cost28, 29. The milestone of 300 Wh g?1 was accomplished by Contemporary Amperex Technology (CATL) (NCM-811, pouch cell), and Panasonic used NCA, 21700 cylindrical cells30. In 2017, China consumed about 72% of EV industry requirements from NCM and NCA, which increased to 90% in 201831.
LiNiO2 (LNO) was first discovered in 1954 by Dyer's group but after LiCoO2 was promoted for LIBs, LNO developed as an emergent cathode due to the low cost of Ni compared to Co 32 . LiNiO2 acquire α-NaFeO2 (space group R3-m) structure forming a closed cubic arrangement with oxide ions and Li+ and Ni3+ employing the octahedral sites with O3 type layered structure.
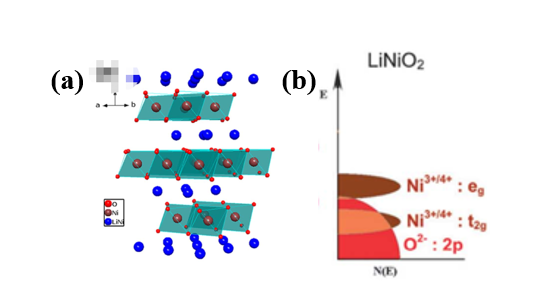
Figure 2: (a) Crystal structure and (b) Energy diagram of LiNiO2. Produced with permission 33. Copyright 2017, Elsevier.
The strong Ni-O-Ni covalency in low spin Ni3+:t2g6 eg1 electronic configuration leads to high conductivity with the rhombohedral crystal structure by sharing their edges to form NiO6 octahedron of trigonal symmetry. Nevertheless, the two-dimensional pathway for lithium diffusion is suitable for lithium conductivity (Figure 2(a)). Moreover, the eg band of Ni3+/4+ and O2-:2p band are non-overlapping, enabling the extraction of one Li+per Ni ion without oxygen loss from the lattice structure as shown in Figure 2(b) 33, 34 . The cell parameters of LiNiO2 (a=2.9 Å, c=14.2 Å with c/a=4.9) are equivalent to the cubic lattice, making the displacement of Li and Ni ions easier compared to LiCoO2 29 `
During high-temperature calcination (about 700 °C), due to the volatilization of lithium, the structure becomes lithium deficient (Li1-xNi1+xO2) with Ni occupying some of the sites of lithium and the difficulty to stabilize all the Ni ions in Ni3+ valence state due to the partial reduction of Ni3+ to Ni2+ will eventually result in low rate capability35, 36. Ohzuku et al. 37 reported that the peak intensity ratio of I (003)/I (104) could be a prime factor to demonstrate the degree of cation mixing and can ultimately predict the electrochemical performance of LiNiO2. However, during the charge/discharge process, LiNiO2 also endures the phase transition, which leads to capacity decay. During the 1st charge cycle, the Ni2+ present in the TM layer gets oxidized; however, with further lithium extraction, Ni2+ in the lithium layer also gets oxidized, resulting in the inter-slab layer contraction38. Dahn et al.39 reported that the electrochemical insertion of additional Li+ into LiNiO2 forms a new Li2NiO2 structure. Thus, during the removal of additional Li+, the redox potential becomes comparatively low resulting in poor structural stability. To resolve the problems of non-stoichiometric Li1-xNi1+xO2, substitution with Co, Mn, and Al are beneficial, which is discussed in the following section.
LiNiO2 is isostructural to LiCoO2, owing to the similar ionic radius of Ni3+ (0.56 Å) with Co3+ (0.545 Å) and the similar electronegativity of Ni (1.91) and Co (1.88) as well as both cathodes require oxygen flow during the synthesis process. Hence, it is beneficial to substitute Ni with Co to minimize cation mixing and suppress the phase transition. The amount of Ni2+ reduces when the substitution of cobalt increases in LiNi1-xCoxO2, and finally, a stoichiometric LiNi0.7Co0.3O2 is synthesized without oxygen flow as reported by Rougier’s group40.
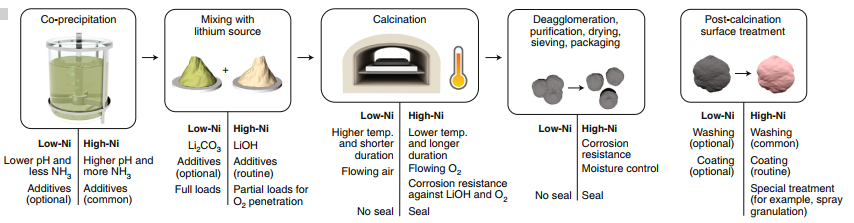
Figure 3: Schematic illustration of manufacturing synthesis of nickel-based layered oxides. Produced with permission8. Copyright 2020, Springer Nature.
In general, the preparation method of Ni-rich cathodes including solvothermal method 46, co-precipitation method47, sol-gel method48, high-temperature solid-state route49, sublimation-induced gas-reacting (SIGR) process50, molten salt method51, microwave-assisted co-precipitation method52, spray drying process53, etc. Among all the synthesis methods, the co-precipitation method can precisely control the morphology at a microscopic level and maintain the elemental distribution. It is also beneficial for large-scale production for industrialization. The preparation steps include mixing the solutions of transition metals in a continuous stirring tank reactor (CSTR) with the precipitator (NaOH is generally preferred). The nucleation process is very high, which results in the formation of microscopic-sized irregular grains. The composition of transition metal elements is designed according to the requirement. In order to obtain the higher density and particle size being uniformly distributed, a chelating agent is required, such as ammonia solution while carefully adjusting the concentration of the chelating agent, pH value (10.5-11.5), reaction temperature (40-60 ºC), stirring rate, feeding rate, and other parameters is crucial. After continuously stirring in a tank reactor for hours, the particles steadily grow and agglomerates to give the desired morphology and elemental composition. The product obtained is a precursor that is further washed, filtered, and dried to obtained Ni-based cathode material. The precursor is further mixed with a lithium source (specifically LiOH and Li2CO3) followed by high-temperature calcination. Liu et al.52successfully prepared LiNi0.85Co0.05Mn0.1O2 via microwave-assisted co-precipitation method. The advanced synthetic process provides the controlled morphology, well-ordered phase structure, high tap density, and lower cation mixing.
However, for high-Ni cathodes (NCM811), the preparation steps become more complex than the preparation process of NCM523, and the equipment should meet the general standards. They also require LiOH as a lithium source instead of Li2CO3, flowing environment should be O2, long-duration for calcination, post calcination treatment, etc. The sintering environment should be strictly controlled by moisture and erosion reaction. The synthesis parameters such as pH, heating temperature, and ammonia solution should also be adjusted carefully according to the Ni content. The usage of more LiOH, calcination duration and O2 flow costs more in high-Ni oxides; however, it balances the cost of cobalt in NCM523. Hence, the price of NCM811 is comparable with NCM523 however is much lower than NCM1118, 54.
Rechargeable batteries are crucial for electric vehicle and other energy storage applications. Lithium-ion batteries are dominant and their demand is increasing with increasing energy demands. Layered oxides as cathode material are beneficial and their cathode chemistries are suitable to faciliatet high-energy-density for commercialization. Ni-rich layered oxide positive electrode material is considered as next-generation cathode material due to high discharge capacity, high working voltage, and low cost for their application in high-energy lithium-ion batteries (LIBs). Efficient and one-step synthesis strategies are the demand for industrialization of Ni-rich cathodes. One-step synthesis strategies are preferred over multiple-step strategies due to the longer synthesis duration and calcination environment, which requires O2 gas. Substitution of cobalt, manganese and aluminum gives rise to LiNixCoyMnzO2 (NCM) and LiNixCoyAlzO2 (NCA) (x+y+z=1) that can effectively compensate for the performance loss.
Recently, researchers are focusing on reducing and eliminating cobalt from Ni-based cathodes as Co is scarce, expensive, and presents ethical issues in mining. Co-free cathodes are placing a next step towards the commercialization of Ni-rich cathodes. However, the absence of Co presents severe challenges in terms of poor cycling stability and electrochemical properties of Ni-rich layered cathodes. High-Ni (Ni> 0.9) cathodes are also a demand to fulfill the driving range of 300 miles per charge and cost-effectiveness due to the low cost of Ni. Nevertheless, Ni content plays a vital role in the structural and electrochemical stability of Ni-rich cathodes. Hence, by overcoming the material defects, the high specific capacity and high energy density of Ni-rich cathode materials can be achieved for its application in next-generation LIBs.
The authors thank the support from the WEmpower Pakistan organization for promoting our research work in STEM research. The authors declare no competing financial interest.
2016. Tracking Clean Energy Progress. IEA .
Yoon, Chong S, Ryu, Hoon-Hee, Park, Geon-Tae, Kim, Jae-Hyung, Kim, Kwang-Ho & Sun, Yang-Kook . 2018. Extracting maximum capacity from Ni-rich Li [Ni0.95 Co0.025 Mn0.025]O2 cathodes for high-energy-density lithium-ion batteries. Journal of Materials Chemistry A 6(9):4126–4132.
Chu, Steven, Cui, Yi & Liu, Nian . 2017. The path towards sustainable energy. Nature Materials 16(1):16–22.
Montoya, Joseph H, Seitz, Linsey C, Chakthranont, Pongkarn, Vojvodic, Aleksandra, Jaramillo, Thomas F & Nørskov, Jens K . 2017. Materials for solar fuels and chemicals. Nature Materials 16(1):70–81.
Fraunhofer, I S E & E A . 2015. Current and future cost of photovoltaics. Long-term scenarios for market development, System prices and LCOE of utility-scale PV systems. Agora Energiewende 82.
Global, E V & Outlook, . 2019. International Energy Agency .
Sun, Y.-K . 2019. High-Capacity Layered Cathodes for Next-Generation Electric Vehicles. ACS Energy Letters 4(5):1042–1044.
Li, Wangda, Erickson, Evan M & Manthiram, Arumugam . 2020. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nature Energy 5(1):26–34.
2013.
Nguyen Duy, Vinh . 2020. Review on the Hybrid-Electric Propulsion System and Renewables and Energy Storage for Unmanned Aerial Vehicles. International Journal of Electrochemical Science 15:5296–5319.
Liu, Wen, Oh, Pilgun, Liu, Xien, Lee, Min-Joon, Cho, Woongrae, Chae, Sujong, Kim, Youngsik & Cho, Jaephil . 2015. Nickel-Rich Layered Lithium Transition-Metal Oxide for High-Energy Lithium-Ion Batteries. Angewandte Chemie International Edition 54(15):4440–4457.
Zeng, Xiaoqiao, Li, Matthew, Abd El?hady, Deia, Alshitari, Wael, Al?bogami, Abdullah S, Lu, Jun & Amine, Khalil . 2019. Commercialization of Lithium Battery Technologies for Electric Vehicles. Advanced Energy Materials 9(27):1900161.
Grunditz, Emma Arfa & Thiringer, Torbjorn . 2016. Performance Analysis of Current BEVs Based on a Comprehensive Review of Specifications. IEEE Transactions on Transportation Electrification 2(3):270–289.
V & B . 2018. Energy, Technologies, Issues and policies for sustainable mobility. Global Data, Green Car Congress.
Whittingham, M S . 1976. Electrical Energy Storage and Intercalation Chemistry. Science 192(4244):1126–1127.
Mizushima, K, Jones, P C, Wiseman, P J & Goodenough, J B . 1981. LixCoO2 (0. Solid State Ionics 3-4(6):171–174.
Blomgren, G E . 2017. The Development and Future of Lithium Ion Batteries. Journal of The Electrochemical Society 164(1):A5019–A5025.
Lu, Jun, Jung Lee, Yun, Luo, Xiangyi, Chun Lau, Kah, Asadi, Mohammad, Wang, Hsien-Hau, Brombosz, Scott, Wen, Jianguo, Zhai, Dengyun, Chen, Zonghai, Miller, Dean J, Sub Jeong, Yo, Park, Jin-Bum, Zak Fang, Zhigang, Kumar, Bijandra, Salehi-Khojin, Amin, Sun, Yang-Kook, Curtiss, Larry A & Amine, Khalil . 2016. A lithium–oxygen battery based on lithium superoxide. Nature 529(7586):377–382.
Anseán, D, González, M, Viera, J C, Álvarez, J C, Blanco, C & García, V . 2013. Evaluation of LiFePO4 batteries for Electric Vehicle applications, 2013 International Conference on New Concepts in Smart Cities: Fostering Public and Private Alliances (SmartMILE). 1–8
Ding, Yuanli, Cano, Zachary P, Yu, Aiping, Lu, Jun & Chen, Zhongwei . 2019. Automotive Li-Ion Batteries: Current Status and Future Perspectives. Electrochemical Energy Reviews 2(1):1–28.
Mwambeleko, Joachim J & Kulworawanichpong, Thanatchai . 2017. Battery electric multiple units to replace diesel commuter trains serving short and idle routes. Journal of Energy Storage 11:7–15.
Jamil, Sidra, Wang, Gang, Yang, Li, Xie, Xin, Cao, Shuang, Liu, Hong, Chang, Baobao & Wang, Xianyou . Suppressing H2–H3 phase transition in high Ni–low Co layered oxide cathode material by dual modification. Journal of Materials Chemistry A 8(40):21306–21316.
Yu, H & Zhou, H . High-Energy Cathode Materials (Li2MnO3-LiMO2) for Lithium-Ion Batteries. The Journal of Physical Chemistry Letters 2013(8):1268–1280.
Drive, U . 2017. US Drive Electrochemical Energy Storage Technical Team Roadmap.
Deng, Jie, Bae, Chulheung, Marcicki, James, Masias, Alvaro & Miller, Theodore . 2018. Safety modelling and testing of lithium-ion batteries in electrified vehicles. Nature Energy 3(4):261–266.
Abraham, K M . 2015. Prospects and Limits of Energy Storage in Batteries. The Journal of Physical Chemistry Letters 6(5):830–844.
Whittingham, M S . 2004. Lithium Batteries and Cathode Materials. Chemical Reviews 104(10):4271–4302.
Zhang, Shu, Ma, Jun, Hu, Zhenglin, Cui, Guanglei & Chen, Liquan . 2019. Identifying and Addressing Critical Challenges of High-Voltage Layered Ternary Oxide Cathode Materials. Chemistry of Materials31(16):6033–6065.
Butt, Annam, Ali, Ghulam, Tul Kubra, Khadija, Sharif, Rehana, Salman, Ayesha, Bashir, Muzaffar & Jamil, Sidra . 2022. Recent Advances in Enhanced Performance of Ni?Rich Cathode Materials for Li?Ion Batteries: A Review. Energy Technology 10(3):2100775.
Fang, Q, Ncm/Nca, & Vs, . 2019. LFP: The Competitive Edge of LFP. . China Battery Enterprise Alliance.
2019. China’s installed battery capacity surges to 56.9 GWh. Inside EVs.
Dyer, Lawrence D, Borie, Bernard S & Smith, G Pedro . 1954. Alkali Metal-Nickel Oxides of the Type MNiO2. Journal of the American Chemical Society 76(6):1499–1503.
Manthiram, Arumugam, Song, Bohang & Li, Wangda . 2017. A perspective on nickel-rich layered oxide cathodes for lithium-ion batteries. Energy Storage Materials 6:125–139.
Kalyani, P & Kalaiselvi, N . 2005. Various aspects of LiNiO2 chemistry: A review. Science and Technology of Advanced Materials 6(6):689–703.
Dahn, J R, Von Sacken, U, Juzkow, M W & Al?janaby, H . 1991. Rechargeable LiNiO2 / Carbon Cells. Journal of The Electrochemical Society 138(8):2207–2211.
Ohzuku, Tsutomu, Ueda, Atsushi, Nagayama, Masatoshi, Iwakoshi, Yasunobu & Komori, Hideki . 1993. Comparative study of LiCoO2, LiNi Co O2 and LiNiO2 for 4 volt secondary lithium cells. Electrochimica Acta 38(9):1159–1167.
Ohzuku, Tsutomu, Ueda, Atsushi & Nagayama, Masatoshi . 1993. Electrochemistry and Structural Chemistry of LiNiO2 (R3m) for 4 Volt Secondary Lithium Cells. Journal of The Electrochemical Society140(7):1862–1870.
Delmas, C, Pérès, J P, Rougier, A, Demourgues, A, Weill, F, Chadwick, A, Broussely, M, Perton, F, Biensan, P H & Willmann, P . 1997. On the behavior of the LixNiO2 system: an electrochemical and structural overview. Journal of Power Sources 68(1):120–125.
Dahn, J R, Von Sacken, U & Michal, C A . 1990. Structure and electrochemistry of Li1±yNiO2 and a new Li2NiO2 phase with the Ni (OH)2 structure. Solid State Ionics 44(1-2):87–97.
Rougier, A, Saadoune, I, Gravereau, P, Willmann, P & Delmasa, C . 1996. Effect of cobalt substitution on cationic distribution in LiNi1 − y CoyO2 electrode materials. Solid State Ionics 90(1-4):83–90.
Rossen, E, Jones, C D W & Dahn, J R . 1992. Structure and electrochemistry of LixMnyNi1−yO2. Solid State Ionics 57(3):311–318.
Zheng, J, Kan, W H & Manthiram, A . 2015. Role of Mn Content on the Electrochemical Properties of Nickel-Rich Layered LiNi0.8-xCo0.1Mn0.1+xO2 (0.0 ≤ x ≤ 0.08) Cathodes for Lithium-Ion Batteries. ACS Applied Materials & Interfaces 7(12):6926–6934.
Aishova, A, Park, G.-T, Yoon, C S & Sun, Y.-K . 1903179. Advanced Energy Materials 2020(4).
Watanabe, Shoichiro, Kinoshita, Masahiro, Hosokawa, Takashi, Morigaki, Kenichi & Nakura, Kensuke . 2014. Capacity fading of LiAlyNi1−x−yCoxO2 cathode for lithium-ion batteries during accelerated calendar and cycle life tests (effect of depth of discharge in charge–discharge cycling on the suppression of the micro-crack generation of LiAlyNi1−x−yCoxO2 particle) Journal of Power Sources 260:50–56.
Shim, J, Kostecki, R, Richardson, T, Song, X & Striebel, K A . 2002. Electrochemical analysis for cycle performance and capacity fading of a lithium-ion battery cycled at elevated temperature. Journal of Power Sources 112(1):222–230.
Cao, Guolin, Yang, Jiachao, Zhu, Jie, Li, Yunjiao, Xi, Xiaoming, Zheng, Junchao & Xiong, Yike . 2020. A solvothermal route to prepare enhanced LiNi0.88Co0.09Al0.03O2 cathode material taking isopropyl alcohol as solvent. Ionics 26(10):5273–5278.
Su, Yuefeng, Chen, Gang, Chen, Lai, Lu, Yun, Zhang, Qiyu, Lv, Zhao, Li, Cong, Li, Linwei, Liu, Na, Tan, Guoqiang, Bao, Liying, Chen, Shi & Wu, Feng . 2019. High-Rate Structure-Gradient Ni-Rich Cathode Material for Lithium-Ion Batteries. ACS Applied Materials & Interfaces 11(40):36697–36704.
Lee, Suk-Woo, Kim, Hyungsub, Kim, Myeong-Seong, Youn, Hee-Chang, Kang, Kisuk, Cho, Byung-Won, Roh, Kwang Chul & Kim, Kwang-Bum . 2016. Improved electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode material synthesized by citric acid assisted sol-gel method for lithium ion batteries. Journal of Power Sources 315:261–268.
Jamil, Sidra, Yu, Ruizhi, Wang, Qun, Fasehullah, Muhammad, Huang, Yan, Yang, Zhenhua, Yang, Xiukang & Wang, Xianyou . 2020. Enhanced cycling stability of nickel-rich layered oxide by tantalum doping. Journal of Power Sources 473:228597.
Kim, Jieun, Lee, Junghwa, Bae, Changgeun & Kang, Byoungwoo . 2020. Sublimation-Induced Gas-Reacting Process for High-Energy-Density Ni-Rich Electrode Materials. ACS Applied Materials & Interfaces12(10):11745–11752.
Liang, Rui, Wu, Zhi-Yong, Yang, Wen-Mao, Tang, Zuo-Qin, Xiong, Guo-Gang, Cao, Yin-Chun, Hu, Su-Rong & Wang, Zhen-Bo . 2020. A simple one-step molten salt method for synthesis of micron-sized single primary particle LiNi0.8Co0.1Mn0.1O2 cathode material for lithium-ion batteries. Ionics 26(4):1635–1643.
Liu, Yong, Yao, Wenli, Lei, Chao, Zhang, Qian, Zhong, Shengwen & Yan, Zhengquan . 2019. Ni-Rich Oxide LiNi0.85Co0.05Mn0.1O2for Lithium Ion Battery: Effect of Microwave Radiation on Its Morphology and Electrochemical Property. Journal of The Electrochemical Society 166(8):A1300–A1309.
Park, Gi Dae & Chan Kang, Yun . 2014. Characteristics of precursor powders of a nickel-rich cathode material prepared by a spray drying process using water-soluble metal salts. RSC Adv. 4(83):44203–44207.
2018. Production and cost analysis of nickel-rich layered cathode materials (in Chinese) CITIC Securities .
Keywords: Layered oxide, Cathode materials, Nickel rich, Lithium ion batteries
Materials Innovations (MI) is an interdisciplinary journal devoted to significant experimental and theoretical findings on the synthesis, structure, charachterization, processing and applications of materials. Materials Innovations is dedicated to publishing peer reviewed novel, cutting edge reports of broad interest to the materials science community.