
Full Html
Vol 3 Issue 3
Exploration of Discotic Liquid Crystals for Applications via Scanning Tunneling Microscope (STM)
Pages: 24-40
Doi: 10.54738/MI.2023.3301
Doi URL: http://doi.org/10.54738/MI.2023.3301
1 Yangtze Delta Region Institute (Huzhou), University of Electronic Science and Technology of China, Huzhou, 313000, China
2 Department of Physics, School of Natural Sciences (SNS), National University of Science & Technology (NUST), Islamabad, 44000, Pakistan
Discotic liquid crystals (DLCs) are a class of liquid crystals that exhibit unique properties that make them attractive for optoelectronic applications. One of their most beneficial characteristics is their charge-carrier mobility, which is facilitated along the stacking axis. This allows for the efficient movement of electrons through the material, making it useful in the construction of electronic devices. DLCs also exhibit self-assembly, self-healing, and solubility in different organic solvents, making them versatile and easy to work with. In this mini-review, we provide an overview of DLCs and their practical applications, with a focus on their charge-carrier mobility (CCM) and alignment. We explore the impact of alignment on the CCM of DLCs and discuss various processing techniques used to achieve alignment, such as zone-casting, Langmuir-Blodgett deposition, solution casting, surface treatment, I.R. irradiation, zone melting, electric field impact, or usage of sacrificial sheets. Understanding and controlling the alignment of DLCs is essential for the successful application of these materials in electronic devices.
Keywords
Discotic liquid crystals (DLCs), Scanning tunneling microscopy, Optoelectronics, Liquid-solid interface, Nanoparticles self-assembly
Discotic liquid crystals (DLCs) are a type of liquid crystal in which the molecules are disc-shaped, with a flat core and a rim that is typically decorated with alkyl chains 1. These molecules self-organize into columnar assemblies that exhibit liquid crystalline behavior, which means that they have properties of both liquids and solids 2. Unlike conventional nematic or smectic liquid crystals, in which the molecules are rod-shaped or plate-like, respectively, the disc-like shape of the DLC molecules allows them to form highly ordered stacks of columns that can exhibit long-range positional and orientational order 3. The properties of DLCs arise from the interactions between the disc-shaped molecules, which include van der Waals interactions, π-π stacking interactions, and hydrogen bonding 4. The disc-like shape of the molecules also leads to the formation of polarized charge transfer (PCT) complexes, in which electrons are transferred from the core to the rim of neighboring molecules 5. These PCT complexes can facilitate charge transport along the stacking direction, making DLCs highly useful in electronic applications. DLCs can exhibit a range of mesophases, or ordered phases of matter, depending on the temperature and concentration of the material 6. These mesophases include columnar hexagonal, columnar rectangular, and columnar oblique phases, among others 1 7. The properties of the mesophase can be tuned by varying the structure of the DLC molecule, as well as the temperature, pressure, and solvent conditions. DLCs have a range of potential applications, including in electronic devices such as solar cells, field-effect transistors, and light-emitting diodes 8. The highly ordered columnar structures of DLCs can facilitate efficient charge transport, while the self-assembly and self-healing properties of these materials make them easy to work with. Additionally, the solubility of DLCs in various organic solvents makes them highly versatile and compatible with a range of processing techniques 9.
DLCs have piqued the interest of numerous researchers since their discovery by Chandrasekhar in 197710. Considerable investigation and research have been conducted to investigate DLCs in optics and electronic devices. A discotic mesogen possesses a central aromatic core surrounded by three to eight flexible chains 9. Nematic and columnar phases are the primary dualistic categories of mesophases in DLC arrangement. Nematic phase discs have an orientational direction, whereas columnar phase discs stack into columns based on their orientation. (Figure 1).] Different research groups comprehensively analyzes the chemical characteristics of DLCs comprising hexagonal, quadrangular, diagonal, helical, or columnar lamellar flexible configurations 11. On the contrary, the initial narrated thermotropic DLCs, indenes and pseudoazulenes, do not possess flexible chains. Primarily, numerous articles have addressed the strategy and significant DLC configurations and their corresponding features 12. The present perspective emphasizes the characteristics of DLCs for their future use in optoelectronic approaches, underlining the latest development in the DLCs explored via STM.
Figure 1: DLCs in (a) nematic and (b) columnar phases areschematic
Generally, DLCs link via effectual π-π columnlike stacks establishing excessive charge-carrier mobility (CCM). In the columnar stacks, the overlapping of molecular orbitals and the degree of order govern the magnitude of CCM 13. The charge transference, i.e., in a 1-dimensional stack system, assemble into an arrangement that facilitates film development, which is beneficial for electronic and optoelectronic devices based on organics materials. Additionally, DLC's liquid peculiarity retains its capability to self-heal the structural imperfections (i.e., grain boundaries), making several square millimeters huge size distinct domains of several micrometers 14. Hence, the useful possessions of DLCs comprise long-range self-gathering (directive), processing ease, solvent solubility, self-healing (dynamics), and increased mobility of charge carriers. Anisotropic development driven by microsegregation between flexible chains and rigid cores, or van der Walls attraction between cores, has been proposed as the mechanism by which columnar mesophases grow. (V.W.) 15, 16 Along the column direction, the core-core distance is typically around 3.5A, whereas the cores center length in adjoining columns is estimated using mesogen size, which generally is about 20-40A 17, 18. The flexible alkyl chains around the cores effectively separate column from adjacent column. As a result of DLC columns' increased conductivity along the column axis, they are referred as quasi 1-D conducting wires. There is no doubt that herringbone packing is valid in calamatic L.C.s, which show 2-D charge transport similar to that seen in pentacene or oligothiophene 19. Additionally, DLCs have a greater orbital overlap than calamatics. The arrangement of the discoid materials' conjugated core determines their electronic properties at the molecular level due to changes in peripheral chains or aromatic cores, which regulate both the solution and the bulk phase self-assembly 20. As a result, the core and substituent structure of the materials impact their overall electrical properties. Besides this, the processing technique affects the supramolecular assemblage of DLCs. A brief overview of DLCs and charge mobilities explored via STM is elaborated in the present perspective. Afterward, we highlight specific features linked with DLCs, i.e., configuration and orientation. Then various processing procedures to attain suitable arrangements for efficient, practically applicable device applications were mentioned explored via. STM.
Previously, different DLCs have been testified having a considerable number of discoid cores comprising triazine, triphenylene, benzene, pyridine, Hexa-azatrinaphthylene (HATNA), diazatriphenylene, Hexa-azatriphenylene, pyrene, dibenzo-napthacene, dodecaazatrianthracene (DATAN), triazatruxene, or triindole, tricycloquinazoline, perylene, coronene diimide, phthalocyanine (Pc), porphyrin, quin-oxalinophenanthrophenazine (TQPP), and Hexa-perihexabenzocoronene (HBC), applicable for various optoelectronic applications 21, 22, 23. The self-assembly and interaction of DLCs were extremely impacted by flexible chains and the central discoid core. For instance, columnar mesophases cannot be formed by substituting six alkylthio or alkyl groups in hexaazatriphenylene. However, mesophase behavior is induced by H-bonding side groups 24. H-bonding networks among benzamide side-chain clusters of a phthalocyanine derivative were attributed to the formation of columnar aggregates defined by numerouss research group 25, 26, 27. The computational studies attribute this behavior to the discoid cores' intermolecular electrostatic potential 28. It shows an important role in the stabilization or destabilization of columnar phases. A position modification of the functional group (i.e., carboxylic acid, nitro group, or methyl carboxylate) in dibenzophenazine DLCs sequence from "upper" to "lateral" of aromatic core intensely disturbs the stability and mesophases 29, 30. Dibenzophenazine and triphenylene derivatives' stability were also influenced due to symmetry. Besides this, the melting temperatures of symmetrical DLCs declined significantly more than their clearing temperatures 30. Even though electron-withdrawing groups are substituted in the discoid core to stabilize the mesophases, the fluorescence quantum yield increases.
The molecular structure of the DLCs, solvent choice, substrate properties, processing parameters, external stimuli, and annealing can all significantly impact the alignment and organization of DLC films 31, 32. Understanding these factors and tailoring them to the desired outcome can lead to highly ordered, aligned, and efficient charge transport in DLC-based devices.
Charge-carrier mobility is an important property of semiconducting materials, including DLCs. It refers to the ease with which charge carriers (electrons or holes) move through the material when an electric field is applied 33. In general, higher charge-carrier mobilities result in more efficient electronic devices, as charge carriers can move more quickly and with less resistance, leading to faster response times and higher electrical conductivity. In DLCs, the charge-carrier mobility is influenced by several factors, including the molecular structure of the material, the nature and distribution of the charge carriers, and the local environment of the charge carriers within the material. The mobility of charge carriers in DLCs is typically determined by experimental techniques such as field-effect transistors (FETs), time-resolved microwave conductivity (TRMC), and space-charge-limited current (SCLC) measurements 34, 35.The mobility of charge carriers in DLCs is generally lower than in conventional inorganic semiconductors, such as silicon. This is due in part to the relatively disordered molecular structure of DLCs, which can impede the movement of charge carriers through the material. However, researchers have developed several strategies to improve the charge-carrier mobility in DLCs, including the use of high-mobility materials, the optimization of the molecular packing and orientation within the material, and the introduction of dopants or other additives to enhance charge transport 36, 37. One promising approach to enhancing charge-carrier mobility in DLCs is the use of columnar self-assembly, where molecules are stacked in a highly ordered manner to create pathways for charge-carrier transport. Another approach involves the use of charge-transport additives, such as small-molecule dopants or polymer blends, which can improve the efficiency of charge-carrier transport within the DLC material.
Overall, the charge-carrier mobility in DLCs is an important property that influences the performance of electronic devices based on these materials. Further research into the underlying mechanisms of charge transport in DLCs and the development of new strategies to enhance charge-carrier mobility will be critical for advancing the field of organic electronics. In organic semiconducting materials, the charge carrier's mobility (CCM) may regulate the appropriateness and compatibility with electronic devices 38. In crystalline constituents, mobility is intensely affected because it possesses dualistic properties. These planar organic compounds (such as anthracene or pentacene) exhibit advanced mobility in amorphous materials because of their well-organized stacking performance 39. Exceptional mobility has been demonstrated in a single crystal device. The main restrictions of this technology are the expense and time required to develop a single crystal. In general, researchers focus and target those systems that self-assemble under wet-processing surroundings such as charge separation in photovoltaic cells, field-effect transistor switching speed, or light-emitting diode intensity (LED) 40, 41. Various computational and experimental modus operandi has been used to determine the CCM in DLCs. These comprise time-of-flight (TOF) procedure, field-effect mobility, steady-state space charge-limited current (SCLC) or (P.R.) pulse radiolysis time-resolved micro-wave conductivity method (PR-TRMC), PR-TRMC governs CCM at the local level and is comparatively unresponsive to imperfections 42, 43 . They show CCM within spatial constrained domains. It was determined that the defect's presence affected the macroscopic values by using the TOF and field-effect procedures. In SCLC, the CCM is determined by examining the IV characteristics of thin organic films that have been compressed between the injecting electrodes. Previously different researchers elaborately discuss the CCM of various DLCs using TOF15 and PR-TRMC techniques 44. According to Van de Craats and Warman, a lower CCM was commonly perceived during the columnar hexagonal phase (C-HP) 45. The alkyl chains through oxygen atom linked core show low CCM compared to those DLCs in which the chains are attached directly via methylene moiety or through the sulfur. To calculate the maximum CCM, eq. 1 is used, centered on preceding PR-TRMC investigational outcomes on porphyrin, coronene, triphenylene, monoimide, azocarboxyldiimido-perylene, peri-hexabenzocoronene and phthalocyanine.
1 ∑μmax.3e-83=n cm2/V s
Here, n represents the numeral of carbon, nitrogen, and oxygen atoms in the core.
Earlier, Mullen and his fellows proved that CCM anticipated is constrained to core size <40 in Hexa-perihexabenzocoronene (HBC). The nature of the substituents associated with discoid core influences supramolecular order, solvability, and, ultimately, CCM of DLCs. The isotropic temperature and interaction between the discoids were lowered near the point of contact. When kept at room temperature, the intramolecular order is maintained at a high level, with a CCM of 0.73 cm2V-1s-1. When methyl benzenethiol-coated gold nanoparticles are introduced to the C-HP, the conductivity of 2,3,6,7,11-(hexakishexyloxy)triphenylene is raised by three orders of magnitude from 10-12 to 10-9 9 Ω-1 cm-1). Gold nanoparticle chains have been shown to increase the conductivity by a factor of three to four (∼10-6 Ω-1 cm-1). TQPP-[SC12H25]4, was found to have a saturation hole mobility of 10-3 cm2 V-1 s-1, with no mesophase. However, TOF hexapentyloxytriphenylene hole mobility in the (C-HP) reliant on temperature and electric-field having CCM upto 2 10-3 cm2 V-1 s-1. Hexahexylthiotriphenylene demonstrated electron and hole mobility in the order of 0.08 cm2 V-1 s-1 in helical C-HP).96. In general, the lower CCM in the C-HP is due to the increased structural and organizational disorder in the columns caused by the side groups' relatively high vibrational modes freedom. The processing method impacts mobility's reliance on the DLC's morphology. The 2, 3, 6, 7, 10, 11-Hexa(hexyloxy)triphenylene gelation with hydrogen-bonded fibrous groups heightens hole mobility from 4.5 x 10-4 to 1.2 x 10-3 cm2 V-1 s-1. Temperature and electric field strength do not affect mobility, which is self-regulating. Few researchers claimed that CCM mobility is restricted due to structural imperfections. A defect-free assembly is considered an ideal surface increase in mobility of up to 15 centimeters per second. It has been projected that electron mobilities for lateral hopping exist at 300 K and that this value increases considerably during higher molecule assembly and orientation 46.
The molecular structure of the DLCs, such as their shape, size, and chemical functional groups, can significantly influence their properties, including their self-assembly, intermolecular interactions, and charge transport 47. For instance, DLCs with elongated, rod-like shapes tend to exhibit a high degree of anisotropy in their molecular alignment, leading to highly ordered structures with excellent charge transport properties. On the other hand, DLCs with bulky or irregular shapes can disrupt the molecular packing, resulting in lower mobilities.
The choice of solvent used for dissolving the DLCs can impact their molecular packing and alignment on the substrate 48, 49. For instance, polar solvents such as water or ethanol tend to favor polar interactions, leading to highly ordered and aligned DLC films. In contrast, nonpolar solvents like chloroform or hexane can promote π-π stacking interactions between the DLCs, leading to more extended molecular packing.
The substrate used for depositing the DLCs can significantly impact their alignment and organization. Substrates with high surface energy, such as metals or oxide surfaces, can promote the formation of highly ordered and aligned DLC films 50. Additionally, surface functionalization with specific chemical groups or self-assembled monolayers can tailor the surface properties to promote specific intermolecular interactions and molecular alignment.
Various processing parameters such as temperature, pressure, concentration, and deposition rate can significantly impact the alignment and organization of the DLCs 51. For instance, higher temperatures can promote molecular diffusion and reorganization, leading to better-aligned films, while high deposition rates can lead to less ordered structures.
External stimuli such as magnetic fields, light, or electric fields can induce molecular reorientation or alignment, leading to highly ordered DLC films 52. For instance, magnetic fields can align paramagnetic DLCs, while light can induce photoisomerization or photodimerization, leading to specific molecular alignment.
Annealing of DLC films can significantly impact their alignment and organization. Annealing can promote molecular diffusion and reorganization, leading to highly ordered and aligned films 53, 54. The annealing temperature and duration can significantly impact the degree of molecular reorientation, with higher temperatures leading to more extended molecular packing and alignment.
Mesophase engineering in DLCs involves controlling the alignment and organization of the molecules in the liquid crystal phase to optimize their properties for various applications 55, 56. There are several methods for mesophase engineering, including the use of additives, surfactants, and external fields.
Surfactants can also be used to modify the properties of DLCs. For example, adding surfactants can induce the formation of different mesophases, such as lamellar or hexagonal phases, depending on the nature of the surfactant and its interaction with the DLC molecules 57. This has been demonstrated in the case of pyrene-based DLCs, where the addition of surfactants led to the formation of hexagonal mesophases with unique optical and electronic properties.
External fields, such as electric or magnetic fields, can also be used to control the alignment and organization of DLC molecules 58. For example, applying an electric field to DLCs can induce a reorientation of the molecules, which can be used to tune their optical and electronic properties. This has been demonstrated in the case of triphenylene-based DLCs, where the application of an electric field led to a reorientation of the molecules and a change in the color of the material.
Overall, mesophase engineering is an important aspect of DLC research, as it allows for the optimization of their properties for various applications such as electronic and optoelectronic devices. Different techniques were employed to formulate DLC mesophases 59. Several research groups have already examined the well-organized coordinated self-assembly of amphiphilic HBC derivatives. Trinitrofluorene (TNF) substituents in amphiphilic HBC derivatives from a few micrometers extended coaxal nanotubular assembly with 16nm diameter 60. The electron donor graphitic stratum of the -stacked HBC is covered via the electron-accepting TNF layer. It enhances photochemical charge carrier generation, resulting in a rapid photoconductive response. The electron-rich discoid doped with TNF generates charge-transfer complexes (CTC), which leads to the formulation of supramolecular assemblies and mesophases. CTC comprises pentakis(phenylethynyl)phenol and TNF having a 1:1 ratio resulted in diverse mesophases having high clearing temperature 61, 62. CTC can be synthesized by adding a TNF acceptor series to mesogen having 5 methoxy clusters with functional tail. There are also a lot of different electron donors and acceptors at the end of the functionalization of fluorinated dendrons, which makes them even more useful.

Figure 2: (a) “Edge-on”, homogeneous planar unidirectional alignment; (b) homeotropic (face-on) alignment
Columnar DLCs tend to align themselves in two distinct arrangements, which are the face-on (or planar) and edge-on (or perpendicular) alignments 63, 64. In the face-on alignment, the disk-shaped molecules are oriented parallel to the substrate or the surface on which they are deposited, with their long molecular axis lying in the plane of the substrate. This arrangement is favored when the substrate surface is hydrophilic and the DLC molecules have polar substituents or functional groups that can interact favorably with the substrate surface. The face-on alignment can also be achieved by shear or flow alignment during the deposition process. On the other hand, in the edge-on alignment, the disk-shaped molecules are oriented perpendicular to the substrate, with their long molecular axis perpendicular to the plane of the substrate. This arrangement is favored when the substrate surface is hydrophobic and the DLC molecules have nonpolar substituents or functional groups that do not interact favorably with the substrate surface 65. The edge-on alignment can also be achieved by using an external magnetic field during the deposition process.
A homogeneous uniaxial planar structure, also known as edge-on, is when the column axis is parallel to the substrate. In the case of homeotropic orientation, the column axis is perpendicular to the substrate. (Figure 2). The homeotropic orientation is applicable for organic photovoltaics or OLED application, but the unidirectional planar shape is optimal for OFET applications. Guillamat and his colleagues proposed two molecular configurations in rectangular columnar organized mesophases 66. They tilt slightly in one of two directions: either perpendicular or parallel to the substrate. The experiment has proven that when DLCs are slowly cooled from the isotropic phase, face-on alignment of primary molecular strata works as a nucleation site for additional self-assembly of perpendicular discs 67. The choice of alignment depends on the specific application and the desired properties of the DLC material. For example, the edge-on alignment can result in higher charge carrier mobility and better electrical conductivity, while the face-on alignment can result in higher photoconductivity and better optical properties.
The capability of switching orientation and structural alignment is difficult to achieve high CCM 68, 69. DLCs have been aligned using a variety of ways, some of which are detailed below
In this method, a thin film's homogeneous and uniform alignment is achieved via controlling the concentration gradient formed between the moving supporting substrate and the nozzle. Different casting parameters such as solvent, temperature, concentration, supply rate, substrate velocity, and nozzle/substrate distance may slightly influence the acquired aligned layers 70, 71. It permits the prearrangement of organic materials homogeneously and consistently. It enables the uniform and constant arrangement of organic materials. Solvent-dissolved DLC is continuously sprayed onto a moving substrate with a temperature gradient using a nozzle. A thin film's homogenous and uniform alignment is attained via controlling the concentration gradient formed between the moving supporting substrate and the nozzle. Different casting parameters such as solvent, temperature, concentration, supply rate, substrate velocity, and nozzle/substrate distance may slightly influence the acquired aligned layers (Figure 3). Zone casting of HBC-C12 on a PTFE layer resulted in forming a layer having a mobility of 1x10-2 cm2 V-1 s-1 72.Jiang H hypothesized columnar configuration direction of HBC-C12 has a tenfold upsurge in photoconductivity over the perpendicular orientation 72. HBC derivative aligned via the zone-casting technique retains optical anisotropy, which is reversibly (switched on/off) through temperature change leading towards molecules reorientation to an orthogonal stacking arrangement from a tilted stacking arrangement.
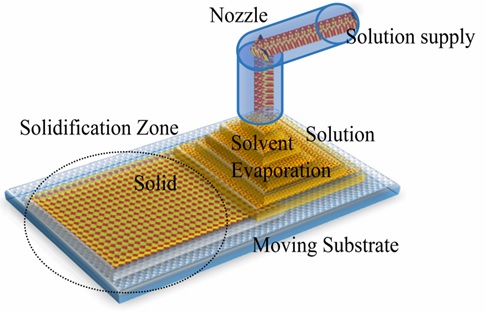
Figure 3: Schematic demonstration of the zone-casting technique
Amphiphilic HBC derivatives were attained via Langmuir-Blodgett (L.B.) Deposition. An L.B. film is formed at the water-air interface when hexaalkylhexa-peri-hexabenzocoronene has an alkyl substituent ended having a carboxylic assembly. The alkyl chain is π-π stacked to generate two separate phases. Another benefit of the benzyl-terminated octa-substituted phthalocyanines is that they create highly well-organized L.B. thin films with high thermal stability, despite the edge-on alignment seen in DLCs synthesized using L.B. (one or two triphenylenes connected to TNF) 73.
This method involves continuously spraying a solvent-dissolved DLC onto a moving substrate with a temperature gradient using a nozzle. By controlling the concentration gradient formed between the moving supporting substrate and the nozzle, a thin film's homogeneous and uniform alignment is achieved. For example, zone casting of HBC-C12 on a PTFE layer resulted in forming a layer having a mobility of 1x10-2 cm2 V-1 s-1. On the substrate, an alternative method for accumulating DLCs involves using oriented polytetrafluoroethylene (PTFE), which can align HBC derivatives such as triphenylene and phthalocyanine 74. An aligned polyimide stratum is also utilized to collect triphenylene DLCs, similar to the previous example.Solution-casting is another method used to study DLCs via STM. In this method, a solution of the DLC material is prepared and then deposited onto a substrate using spin coating or drop casting techniques. The DLC material in the solution aligns with the substrate and forms a thin film. One approach to achieving alignment in solution-casting is using an oriented polytetrafluoroethylene (PTFE) substrate. PTFE has a high surface energy, which enables it to align HBC derivatives such as triphenylene and phthalocyanine. The DLC material is dissolved in a suitable solvent and then deposited onto the PTFE substrate using spin coating or drop casting techniques. The PTFE substrate aligns the DLC molecules, resulting in a thin film with a preferred orientation.
Another approach involves using an aligned polyimide layer to collect triphenylene DLCs 75. The polyimide layer is first aligned by rubbing it with a velvet cloth or using a photoalignment technique. The DLC material is then deposited onto the aligned polyimide layer using spin coating or drop casting techniques. The DLC molecules align with the polyimide layer and form a thin film with a preferred orientation 76. Overall, solution-casting is a versatile method for preparing DLC thin films with various orientations and alignments. It allows for the deposition of DLCs onto a wide range of substrates and can be used to study the structure and properties of DLCs using various techniques, including STM.
Annealing is a widely used treatment for aligning DLCs, where the material is heated above its crystallization temperature and then cooled at a controlled rate to form stable thin coatings 77. The use of surface treatments such as Ar plasma, UV-ozone, or annealing on an ITO surface can produce well-aligned DLC thin films, which can be favorable for wet conditions and organic thin film formation. In addition, different surface and interface interactions can influence the configuration of DLCs. One example is the use of phosphocyanine (Pc) derivatives with octet oligo(ethyleneoxy) peripheral substituents, which have been found to exhibit different alignments depending on the surface. On a hydrophobic surface, the rod-like liquid crystalline molecule aligns perpendicularly to the substrate to achieve homeotropic alignment 78. In contrast, when the Pc molecule's outer chains have diverse interfaces, such as those with hydrophilic surfaces, this results in homogeneous or planar alignment, where the molecules align parallel to the substrate. Another treatment method for modifying the alignment of DLCs is through circularly polarized infrared (IR) irradiation. This technique activates the vibrational mode of a mesogen's particular chemical bond, allowing for a specific configuration to be attained based on the incident photon polarization direction and transition dipole moment for the vibrational excitation. This alignment is typically achieved in higher-ordered DLC phases, such as the Colh mesophase, which has a high viscosity. Overall, surface treatments and annealing can significantly influence the alignment and configuration of DLCs, and the use of circularly polarized IR irradiation can provide a means for selectively modifying the alignment of specific chemical bonds in the mesogens. These techniques are important for exploring the potential applications of DLCs in optoelectronics, where precise alignment is critical for efficient device performance.
Infrared (IR) irradiation is a technique used to modify the configuration of DLCs for their effective utilization in optoelectronics 79. The alignment of DLCs can be changed by activating the vibrational mode of a mesogen's specific chemical bond using circularly polarized infrared irradiation. The vibrational excitation is achieved by a specific grouping of the incident photon polarization direction and transition dipole moment 80. This technique is only effective in higher-ordered DLC phases with high viscosity, such as the Colh mesophase. The use of IR irradiation to modify the alignment of DLCs has been reported in studies by Shimizu and colleagues 50. By changing the alignment of DLCs using IR irradiation, it is possible to optimize their charge-carrier mobility and other properties for use in optoelectronic applications. However, the precise mechanism of this alignment process and the optimal conditions for achieving it are still the subject of ongoing research. Circularly polarized infrared irradiation technique is utilized to modify the configuration of the DLCs. Polarized infrared irradiation changes the DLC alignment by activating the vibrational mode of a mesogen's particular chemical bond. The specific grouping of the incident photon polarization direction and transition dipole moment for the vibrational excitation permits attaining a particular configuration.
Annealing is a successful way of aligning the DLCs 81. For example, an ITO surface treatment using Ar plasma, annealing, or UV-ozone can produce stable DLC thin coatings. The DLC material is heated above its crystallization temperature and then cooled at a regulated degree to manufacturing these films.
In this method, an L.B. film is formed at the water-air interface when hexaalkylhexa-peri-hexabenzocoronene has an alkyl substituent ended having a carboxylic assembly. The alkyl chain is π-π stacked to generate two separate phases. Benzyl-terminated octa-substituted phthalocyanines can create highly organized L.B. thin films with high thermal stability.
The mechanism to study DLC via STM influence involves the use of a magnetic field to achieve a uniaxial orientation of the discotic metallomesogens 82. In this case, CoS12 DLC was used, which is a diamagnetic material that maintains its interaction even in the presence of an external magnetic field. Lee and colleagues were able to achieve a magnetic uniaxial columnar assemblage of the CoS12 DLC by continuously rotating the sample from isotropic phase to liquid crystalline phase while in the presence of a static magnetic field. The external magnetic field provided an orientation for the DLC, aligning it edge-on. This alignment allowed for the DLC to be studied using STM, which can visualize the surface of the material at the atomic level. This technique is useful for understanding the morphology and structure of DLCs, which is important for their optoelectronics applications. Magnetic alignment is just one of the methods that can be used to achieve alignment of DLCs, and other methods such as surface treatment, zone casting, and Langmuir-Blodgett deposition have also been explored.
Various surface and interface interactions can change the DLCs' configuration 83. For example, phosphocyanine derivatives connected to octet oligo(ethyleneoxy) peripheral substituents on a hydrophilic and homogenous orientation on a hydrophobic surface have previously exhibited different alignments. To study discotic liquid crystals (DLCs) using scanning tunneling microscopy (STM), it is important to first prepare the sample in a way that allows for proper alignment and observation of its properties 84. One method to achieve alignment is through the use of a sacrificial polymer layer. This method has been demonstrated in an alkoxy phthalocyanine DLC where homeotropic alignment was achieved. The first step in this process involves spin coating or casting a layer of sacrificial polymer onto a substrate. The DLC is then placed on top of the sacrificial layer. The sacrificial layer acts as a temporary substrate that can be removed once the DLC is properly aligned. To align the DLC, thermal annealing is used to induce homeotropic alignment. The sample is heated to a specific temperature to induce the desired alignment, and then cooled to ambient temperature to maintain the alignment. Once the alignment is achieved, the sacrificial layer can be removed through a process known as lift-off. This involves dissolving the sacrificial layer in a solvent that does not affect the DLC. Using a sacrificial layer makes it possible to achieve homeotropic alignment of DLCs, which allows for observation of their properties using STM. This method has been used to study various DLCs and is a valuable tool for developing optoelectronic devices.
Scanning Tunneling Microscopy (STM) is a powerful tool for investigating the structure and properties of materials at the nanoscale, including Discotic Liquid Crystals (DLCs) 85. DLCs exhibit unique properties that make them attractive for use in optoelectronic applications, such as charge-carrier mobility, self-assembly, and solubility in different organic solvents. STM allows for the visualization of DLCs at the molecular level, providing insight into the structure and arrangement of the disc-shaped molecules. STM imaging can reveal the formation of ordered columnar structures, each consisting of stacked discotic molecules. These columns can be aligned in various orientations, depending on the processing conditions used to prepare the DLC material.
STM can also be used to study the charge transport properties of DLCs. By measuring the tunneling current between the STM tip and the DLC material, it is possible to investigate the movement of charge carriers along the columns of discotic molecules. The charge-carrier mobility of DLCs can be affected by a range of factors, including the alignment of the columns and the presence of defects or impurities in the material. In addition to imaging and charge transport measurements, STM can also be used to manipulate and modify the structure of DLCs. For example, the tip of the STM can be used to apply an electric field to the material, which can induce changes in the alignment of the discotic columns. This can be used to create patterns or structures in the DLC material, which can be useful for constructing electronic devices. Hence, STM is a valuable tool for investigating the structure and properties of DLCs, providing insight into their potential applications in the field of optoelectronics. By understanding the behavior of these materials at the nanoscale, it is possible to develop new and innovative electronic devices that harness the unique properties of DLCs.
Lee and his colleagues suggest an alternate technique for phase-control and electrical measurements of DLCs at the nanoscale 51. Using STM and STS, they investigate the discotic liquid crystalline of ndibenzo[a, c]phenazine and the electron transfer characteristics of self-assembled monolayers formed by a DLC molecule at liquid-solid (L.S.) boundary. As targeted molecules possess an internal dipole moment, the polarity of the substrate underneath may regulate the molecular polymorphs. They observed two types of orientation, head-to-head and non-head-to-head, which were resistant to negative and positive STM bias. An STM tip may modify their transitions locally as shown in Figure 4(a).Besides this the solvent did not impact the morphology and orientation of molecules. It is due to the low polarity or lack of solvents, co-adsorbed molecules are unaffected by the solvent. Unlike in 2D systems, where substrate effects play an important role, 3D systems emphasize intermolecular attractions, which leads to columnar packing (Figure 4(b). Additionally, STS results demonstrate that the bandgap of packing I was approximately 400 mV smaller than that of packing II. The STM contrast of packing I is brighter than packing ii (Figure 4(c). According to STM, a brighter contrast indicates higher conductivity, which is in line with this concept. Octanoic acid or n-tetradecane molecular solvent did not alter surface packing in any substantial way. There is an insignificant solvent effect on self-assemblies in a 2D self-assembly system compared to those seen in a 3D design.
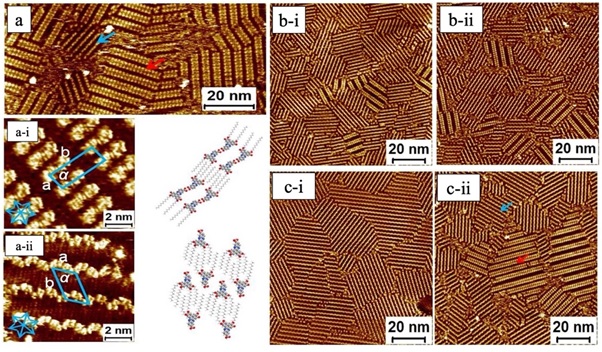
Figure 4: (a) STM images of self-assembly of 1 using 1.0-mM sample solution. a (i, ii) STM image and models of packing-i and packing-ii. STM image of solvent effect:b (i) octanoic acid, (ii) n-tetradecane. c (i, ii) STM images of concentration-dependent surfaces comprised of the polymorphs of 1. The concentration of sample solutions for (i): 0.01 mM; (ii): 0.5 mM. The red and blue arrows in b) indicate the packing-i and packing-ii motifs, Imaging conditions: Ebias, -900 mV; itunnelling, 150 pA. Reprinted with permission 51.
Hence, molecules' properties, such as optical, mechanical, heterogeneous catalysis and selectivity, can be influenced by their molecular arrangement, as shown in this study. Numerous 2D materials consisting of self-assembly films have shown fascinating phenomena. Chunli Bai and his colleagues investigate 1,7,13-trialkanoyldecacyclene [TTD](with n-carbon side chains, n=14 & 18) 52. According to their research, there is a way to fill two dimensions of a triphenylene core with only three side chains at the interface. This study might serve as a counter-example to previous research that found that three elongated chains would not be enough to occupy the center's area. Figure 5 shows an STM view of a stratum of TTD on a graphite substrate, where two orientation patterns represent the zigzag motifs, as clearly evident. The observed domains have darker and brighter regions, corresponding positions of interdigitated alkyl chains and decacyclene core moieties, respectively.
The TTD molecules were assembled into supramolecular twin chains stabilized by 2D alkyl crystallization. The assembling configurations seen might be explained by the influence of two-dimensional alkane lamella and steric barrier caused by adsorbate-substrate interactions. This could result in a loss of symmetry in nonchiral molecules. Specific geometries favored energetically resulting in structural transformations, i.e., chirality or the disintegration of molecular symmetry, are two examples of this. They can be interpreted in terms of three different driving forces. The initial step is to attain the best possible alignment with the substrate lattice. The second goal is to encourage the generation of monolayers in which hydrogen atoms are thought to be in equilibrium. The steric hindrance encourage molecules to attain antiparallel directions, i.e., antiferromagnetic-like triangular networks. Besides this, alkyl chains acquire parallel orientations to achieve maximum local 2D crystallization regardless of the core molecular symmetries.s. As a matter of fact, molecules may experience spontaneous symmetrical splitting and lose their previous configuration. Decacyclene derivatives would have more steric hindrance due to their larger cores than triphenylene derivatives, and longer side chain would result in more 2D crystallization. In a two-dimensional system, the TTD configuration appears to balance core-substrate intermolecular interactions and surface free energy minimization. A similar arrangement is used by TTD when four additional methylene units are added; however, due to their longer side chains, the separation and angle were altered. HDTP, a triphenylene derivative with only 12 methylene side chains, retains the uniformity of organic molecules through self-assembly.
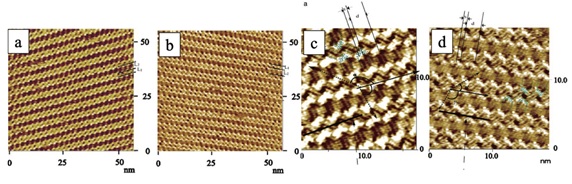
Figure 5: A large-scale image of one of two orientated stripes (57 ×57 nm2). Tunneling conditions: 800 mV, 1.10 nA. (b) STM image of the other of two orientated stripes (57 × 57 nm2). Tunneling conditions: 880 mV, 0.99.(c,d) High-resolution STM images of the TTD assembly. Reprinted with permission 52.
Due to "their self-assembling characteristics and innovative structural features, liquid crystals are attracting interest from experts in the development of organic light-emitting devices, photovoltaics, and thin-film transistors, along with other scientific disciplines. The polar packing of molecules gives rise to Ferro-or antiferroelectric properties having numerous practical applications, such as piezoelectricity, pyroelectricity, and fast-switching electrooptical devices. It has been predicted that the transition temperature would decrease with increasing thickness. Still, in a study by Bune et al., 53a first-order ferroelectric phase was found with a transition temperature nearly identical to the bulk value, challenging this prediction antiferroelectric L.C. phase's structure was demonstrated by an STM image, which revealed that the layers are made up of innovative quadruple "zigzag" molecular rows. STM is used to investigate the submolecular structures of 2D assembly of banana-shaped LC molecules, P-n-PIMB (n) 18, 14, and 12, on the HOPG substrate. The findings reveal two types of coexisting molecular arrangements. This surface nano-sized assembling behavioral patterns of liquid crystals may give important information for experimental application and theoretical research 54, 55, 56, 86, 57, 58, 59.
The scanning tunneling microscope (STM) is a powerful tool that uses an external electric field to control chemical reactions and supramolecular phase transitions at molecular scales. However, it has not been widely used for crystal engineering applications. Khan, S.B and his colleagues demonstrate the use of the directional electric field of an STM to manipulate supramolecular crystallization on a solid surface 87. They found that a random-tiling assembly of p-terphenyl-3,5,3’,5’-tetracarboxylic acid can be transformed into close-packed periodic assemblies under positive substrate bias conditions at the liquid/solid interface. They were able to tailor the nucleation and crystal growth of the resulting field-induced products in real-time, producing a two-dimensional supramolecular single crystal. The crystals exhibited bright spectroscopic features that were strongly dependent on the STM bias, corresponding to variations in electron density of states. As shown in Figure 6(a), the chemical structure of a TPTC target molecule. While the core A–E of the random tiling displays a negative STM bias, all other closed packed patterns are biased positively. The R and S forms are mirror images of one another at the macro level, and the H (head-to-head) package is a likely third form, all of which share the same cell unit size. The normal direction (n line) of TPTC is perpendicular to the packaging axis (m line) of the close packing motif, which runs in the same plane as HOPG. The blue arrows represent the path of the graphite structure of the grid. The Ebias and itunneling are 0.5 V, 70 pA. The d) and e) panels show the random tiling of the five pores on the surface and the percentage of them. Parameters of the unit cells of a, b, and α: 0,9 (± 0,3) nm. They report that an external, orientated electric field of STM can be utilized to generate STM bias-related new 2D self-assembled structures, which would be otherwise inaccessible by the drop-casting or spin-coating methods.
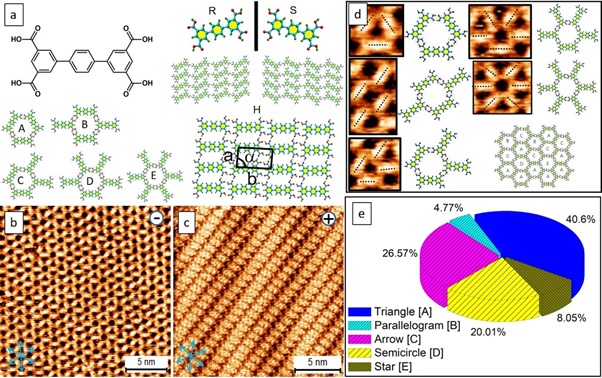
Figure 6: TPTC target molecule chemical structure. (b & c)The close-packed phase features a mirror image (R and S forms) and an expected H (head-to-head) packing with the same unit cell dimensions. The packing axis of the close packing motif goes along one of the normal directions of HOPG below (m line), and a molecular skeleton is 60o from the packing axis so that TPTC lies nearly along another normal direction (n line). Graphite lattice structural direction is shown by blue arrows. 0.5 V, 70 pA for imaging (Ebias, itunneling). (d) & (e) demonstrate the random tiling and surface pores. a, b, and α unit-cell parameters: 0.9 (± 0.3) nm, 1.7 (± 0.3), 87° (± 2°). Reprinted with permission 87
DLCs have shown great potential in optoelectronics applications due to their unique electronic and optical properties. Here are some of the optoelectronics applications of DLCs. Figure 7 displays the DLCs applications in numerous domains.
DLCs can be used as emissive and conductive layers in OLEDs due to their high carrier mobility, high thermal stability, and strong charge transfer ability. This results in efficient light emission and better device performance 88.
DLCs can be used as active layers in OSCs due to their good photoconductivity and high electron mobility. They can also be used as hole blocking and electron blocking layers in OSCs to improve device efficiency 89.
DLCs can be used as semiconducting layers in OFETs due to their high carrier mobility and low operating voltage 90. This results in better device performance and low power consumption.
DLCs can be used as photoactive layers in photovoltaic devices due to their high light absorption coefficient and high photoconversion efficiency 91. They can also be used as charge transport layers to improve device performance.
DLCs can be used as sensitive layers in optoelectronic sensors due to their high sensitivity and selectivity towards specific analytes 92. They can also be used as transducers to convert optical signals into electrical signals.
DLCs can be used as semiconducting layers in OFETs due to their high carrier mobility and low operating voltage. This results in better device performance and low power consumption.
DLCs can be used as photoactive layers in photovoltaic devices due to their high light absorption coefficient and high photoconversion efficiency 93. They can also be used as charge transport layers to improve device performance.
DLCs can be used as sensitive layers in optoelectronic sensors due to their high sensitivity and selectivity towards specific analytes 60. They can also be used as transducers to convert optical signals into electrical signals.
There are various types of DLCs, including hydrogenated DLC (a-C:H), tetrahedral amorphous carbon (ta-C), nitrogenated DLC (a-C:N), and fluorinated DLC (a-C:F) 94, 95. Each type has its unique properties and potential applications in various fields. Here are some examples:
HBC is a columnar DLC that has been used in organic light-emitting diodes (OLEDs) as an electron transport material. It has a high charge carrier mobility and can improve the efficiency of OLEDs.
Pc is a flat DLC that has been used in organic photovoltaics (OPVs) as a donor material. It has a strong absorption in the visible and near-infrared regions, making it useful for harvesting solar energy. Pc derivatives can also be used as electron transport materials in OLEDs.
PDI is a planar DLC that has been used in OPVs as an acceptor material. It has a high electron affinity and can improve the efficiency of OPVs. PDI derivatives can also be used as hole transport materials in OLEDs.
TP is a columnar DLC that has been used in OLEDs as a hole transport material. It has a high charge carrier mobility and can improve the efficiency of OLEDs.
Tetracene is a planar DLC that has been used in organic field-effect transistors (OFETs) as an active material. It has a high charge carrier mobility and can be used for high-performance electronic applications.
PDI derivatives are a class of DLCs that have been widely studied for their potential use in organic solar cells. They exhibit good electron transport properties and high electron mobility, which can improve the efficiency of solar cells.
Quinacridone derivatives are another class of DLCs that have been used in organic solar cells. They have a high extinction coefficient and can absorb light over a wide range of wavelengths, making them useful for harvesting solar energy.
Phthalocyanine derivatives are a versatile class of DLCs that have been used in a variety of optoelectronic applications, including organic solar cells, organic light-emitting diodes (OLEDs), and photovoltaic devices. They have a high absorption coefficient and can be easily functionalized to tune their optical and electronic properties.
Triphenylamine derivatives are a type of DLC that have been used in OLEDs due to their high hole mobility and good charge injection properties. They have also been used in organic photovoltaics as electron donors.
SubPc is a DLC that has been used in organic photovoltaics due to its high electron mobility and good stability. It has also been used in OLEDs and other optoelectronic devices.
Figure 7: DLCs applications and benefits in different domains.
DLCs have attracted significant attention recently due to their unique properties, including high charge-carrier mobility along the stacking axis, self-assembly, self-healing, and solubility in various organic solvents 96. These properties make DLCs promising candidates for optoelectronic applications, such as solar cells, organic light-emitting diodes, and field-effect transistors 97. The use of STM in exploring the structure and properties of DLCs has emerged as a promising approach for developing efficient electronic devices. STM allows for the visualization of DLCs at the molecular level, providing insight into the structure and arrangement of the disc-shaped molecules. STM imaging can reveal the formation of ordered columnar structures, with each column consisting of stacked discotic molecules. STM can also be used to study the charge transport properties of DLCs and manipulate their structure to create patterns or structures useful for electronic device construction. Overall, exploring DLCs for optoelectronics applications via STM holds great potential for developing innovative electronic devices 98, 99.
As recent literature explains, this perspective describes the design, synthesis, and morphology of numerous ILCs. Columnar L.C.s provide a variety of ways of achieving tailored structural spiral assemblies. The self-organization process comprises stacking units, self-assembly within twisting columns, and column orientation within prescribed 2D lattices. The non-covalent contacts interact so that the spiral columnar architecture is formed. Another functional property associated with these helical structures is supramolecular chirality, which can be reversibly altered by externally driving the twisted sense. This enables the development of information frameworks of columnar mesophases to switch their chirooptical characteristics 100. Thus, the ultimate goal is to discover how to manage molecular self-assembly and develop novel colloidal systems with specified functions or features closely related to the desired applications through attractive forces. This is an interdisciplinary approach, as the breadth of this research extends considerably beyond the typical boundaries of organic chemistry. This technology applies to various fields, including cosmetics, pharmaceutical formulations, nanomaterials, and catalysis. Additionally, this enables the production of intermediary systems between homogeneous and heterogeneous mixes, combining the features of homogeneous and heterogeneous catalysis. Consequently, mass transfer between the catalyst and the substrates is significantly enhanced by the water/oil contact, and no stirring is necessary. The separation of reactants and products prevents (or at least reduces) side reactions from occurring 101. Additionally, nanotube films can be assembled using established methods of L.C. alignment, such as grooved surfaces, magnetic fields, and patterned electrodes, to control their organization. The nematic matrix is found to order single- and multi-walled carbon nanotubes in L.C. solvents. Other nanometer-sized building blocks can be aligned with L.C. to control organized nanomaterials and their structures. The next stage in developing usable materials and functioning devices is to invent processing methodologies that allow the controlled assemblage of configurations into larger systems. Single- and multi-walled nanocomposites (SWCNT and MWCNT) have been showing promise building blocks for a broad array of applications, so processes for organizing them are particularly interesting.
In conclusion, DLCs have emerged as promising candidates for optoelectronic applications, with their unique properties such as high charge-carrier mobility along the stacking axis, self-assembly, and solubility in various organic solvents. The use of Scanning Tunneling Microscopy (STM) in exploring the structure and properties of DLCs has provided valuable insights into the behavior of these materials at the nanoscale. By visualizing DLCs at the molecular level and measuring the tunneling current between the STM tip and the DLC material, STM has enabled the investigation of the charge transport properties of DLCs and their potential for use in electronic devices. The manipulation of DLCs using STM has also allowed for creating patterns or structures useful for electronic device construction. Overall, exploring DLCs for optoelectronics applications via STM holds great potential for developing efficient and innovative electronic devices.
Exploring discotic liquid crystals (DLCs) for optoelectronic applications through scanning tunneling microscope (STM) provides a promising avenue for developing novel electronic devices. STM enables the observation of molecular arrangement and electronic properties of DLCs at the nanoscale level. Through the manipulation of the DLCs' self-assembly process, it is possible to control their morphology, such as column orientation within prescribed 2D lattices, and tailor their properties for specific applications. The alignment of DLCs through established methods such as zone-casting, Langmuir-Blodgett deposition, or electric field impact, allows for the control of their electronic properties and charge-carrier mobility (CCM). STM also enables the study of DLCs' supramolecular chirality, which can be reversibly altered by driving the twisted sense. It opens the possibility of developing information frameworks to switch their chirooptical characteristics. The interdisciplinary approach required for developing DLCs for optoelectronic applications extends beyond the typical boundaries of organic chemistry. It applies to various fields such as cosmetics, pharmaceutical formulations, nanomaterials, and catalysis. In conclusion, the use of STM in exploring DLCs offers a promising avenue for developing functional materials and devices for optoelectronic applications.
Conflict of Interest
The authors declare no conflict of interest.
Lee, Kum Hee, Kim, Seul Ong, Kang, Sunwoo, Lee, Jin Yong, Yook, Kyoung Soo, Lee, Jun Yeob & Yoon, Seung Soo . 2012. Indenofluorene-Based Blue Fluorescent Compounds and Their Application in Highly Efficient Organic Light-Emitting Diodes. European Journal of Organic Chemistry 2012(14):2748–2755.
Vinayakumara, D R, Swamynathan, K, Kumar, Sandeep & Adhikari, Airody Vasudeva . 2018. Optoelectronic exploration of novel non-symmetrical star-shaped discotic liquid crystals based on cyanopyridine. New Journal of Chemistry 42(20):16999–17008.
Lee, Seung Joon, Park, Jin Su, Yoon, Kyung-Jin J, Kim, Young-Inn I, Jin, Sung-Ho H, Kang, Sung Kwon, Gal, Yeong-Soon S, Kang, Sunwoo K, Lee, Jin Yong, Kang, Jae-Wook K, Lee, Se-Hyung J, Park, Hyung-Dol S & Kim, Jang-Joo I . 2008. High-Efficiency Deep-Blue Light-Emitting Diodes Based on Phenylquinoline/Carbazole-Based Compounds. Advanced Functional Materials 18(24):3922–3930.
Nehring, Austin, Shendruk, Tyler N & De Haan, Hendrick W . 2018. Morphology of depletant-induced erythrocyte aggregates. Soft Matter 14(40):8160–8171.
Keum, Changmin, Becker, David, Archer, Emily, Bock, Harald, Kitzerow, Heinz, Gather, Malte C & Murawski, Caroline . 2020. Organic Light?Emitting Diodes Based on a Columnar Liquid?Crystalline Perylene Emitter. Advanced Optical Materials 8(17):2000414.
Zhao, Ke-Qing Q, Jing, Min, An, Ling-Ling L, Du, Jun-Qi Q, Wang, Yan-Hong H, Hu, Ping, Wang, Bi-Qin H, Monobe, Hirosato, Heinrich, Benoît & Donnio, Bertrand . 2017. Facile transformation of 1-aryltriphenylenes into dibenzo[fg,op]tetracenes by intramolecular Scholl cyclodehydrogenation: synthesis, self-assembly, and charge carrier mobility of large π-extended discogens. Journal of Materials Chemistry C5(3):669–682.
Maeda, Hiromitsu, Okubo, Takayuki, Haketa, Yohei & Yasuda, Nobuhiro . 2018. Pyrrole-Based Zwitterionic π-Electronic Systems That Form Self-Assembled Dimers. Chemistry - A European Journal24(60):16176–16182.
Volpi, Riccardo, Camilo, Ana Claudia Santos, Filho, Demetrio A Da Silva, Navarrete, Juan T López, Gómez-Lor, Berta, Delgado, M Carmen Ruiz & Linares, Mathieu . 2017. Modelling charge transport of discotic liquid-crystalline triindoles: the role of peripheral substitution. Physical Chemistry Chemical Physics 19(35):24202–24208.
Kim, Kwon-Hyeon H & Kim, Jang-Joo H . 2018. Origin and Control of Orientation of Phosphorescent and TADF Dyes for High-Efficiency OLEDs. Advanced Materials 30(42):1705600.
Keum, Changmin, Becker, David, Archer, Emily, Bock, Harald, Kitzerow, Heinz, Gather, Malte C & Murawski, Caroline . 2020. Organic Light?Emitting Diodes Based on a Columnar Liquid?Crystalline Perylene Emitter. Advanced Optical Materials 8(17):2000414.
Rasool, Shafket, Kim, Jae Won, Cho, Hye Won, Kim, Ye?jin W, Lee, Dong Chan, Park, Chan Beom, Lee, Woojin C, Kwon, Oh?hoon H, Cho, Shinuk W & Kim, Jin Young . 2023. Morphologically Controlled Efficient Air?Processed Organic Solar Cells from Halogen?Free Solvent System. Advanced Energy Materials 13(7):2203452.
Cui, Yong, Xu, Ye, Yao, Huifeng, Bi, Pengqing, Hong, Ling, Zhang, Jianqi, Zu, Yunfei, Zhang, Tao, Qin, Jinzhao, Ren, Junzhen, Chen, Zhihao, He, Chang, Hao, Xiaotao, Wei, Zhixiang & Hou, Jianhui . 2021.Single?Junction Organic Photovoltaic Cell with 19% Efficiency. Advanced Materials 33(41):2102420.
Zhu, Tao, Zheng, Luyao, Xiao, Zuo, Meng, Xianyi, Liu, Lei, Ding, Liming & Gong, Xiong . 2019. Functionality of Non?Fullerene Electron Acceptors in Ternary Organic Solar Cells. Solar RRL 3(12):1900322.
Li, Y . 2013. Fullerene-Bisadduct Acceptors for Polymer Solar Cells. Chemistry - An Asian Journal 8(10):2316–2328.
Koh, Chang Woo, Cho, Hye Won, Rashid, Md Al Mamunur, Lee, Tack Ho, Park, Song Yi, Lee, Wonho H, Kwak, Kyungwon, Kim, Jin Young & Woo, Han Young . 2020. 2D Star?Shaped Non?Fullerene Electron Acceptors with Modulation of J?/H?Type Aggregations: Molecular Design–Morphology–Electrical Property Correlation. Advanced Materials Technologies 5(6):2000174.
Zellman, Carson O, Vu, Danielle & Williams, Vance E . 2020. Adjacent functional group effects on the assembly of columnar liquid crystals. Canadian Journal of Chemistry 98(7):379–385.
Lehmann, Matthias, Dechant, Moritz, Lambov, Martin & Ghosh, Tapas . 2019. Free Space in Liquid Crystals—Molecular Design, Generation, and Usage. Accounts of Chemical Research 52(6):1653–1664.
Vishwakarma, Vinod Kumar & Sudhakar, Achalkumar Ammathnadu . 2021. Structure–property relationships of quinoxaline-based liquid crystals. Soft Matter 17(36):8221–8257.
Zellman, Carson O & Williams, Vance E . 2023. Strategies for promoting discotic nematic phases. Soft Matter 19(15):2705–2709.
Lu, Yang, Li, Yaming, Zhang, Rong, Jin, Kun & Duan, Chunying . 2013. Regioselective ortho-nitration of N-phenyl carboxamides and primary anilines using bismuth nitrate/acetic anhydride. Tetrahedron69(45):9422–9427.
He, Rongjun, Bai, Yunpeng, Yu, Zhi-Hong H, Wu, Li, Gunawan, Andrea Michelle & Zhang, Zhong-Yin Y . 2014. Diversity-oriented synthesis for novel, selective and drug-like inhibitors for a phosphatase from Mycobacterium tuberculosis. Med. Chem. Commun. 5(10):1496–1499.
Anthony Bozek, K J, Ho, K I, Saint-Martin, T, Argyropoulos, P & Williams, V E . 2015. Liquid crystalline esters of dibenzophenazines. Materials 8:270–284.
Foster, E Johan, Jones, R Bradley, Lavigueur, Christine & Williams, Vance E . 2006. Structural Factors Controlling the Self-Assembly of Columnar Liquid Crystals. Journal of the American Chemical Society128(26):8569–8574.
Foster, E Johan, Lavigueur, Christine, Ke, Ying-Chieh C & Williams, Vance E . 2005. Self-assembly of hydrogen-bonded molecules: discotic and elliptical mesogens. Journal of Materials Chemistry 15(37):4062.
Lavigueur, Christine, Foster, E Johan & Williams, Vance E . 2008. Self-Assembly of Discotic Mesogens in Solution and in Liquid Crystalline Phases: Effects of Substituent Position and Hydrogen Bonding. Journal of the American Chemical Society 130(35):11791–11800.
Cammidge, A N & Jackson, D J B . 2006. The effect of size and shape variation in discotic liquid crystals based on triphenylene cores. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 364(1847):2697–2708.
Lavigueur, Christine, Foster, Johan E & Williams, Vance E . 2007. Modular assembly of elliptical mesogens. Liquid Crystals 34(7):833–840.
Yoshida, Jun, Bozek, Kevin J A, Thompson, John R & Williams, Vance E . 2019. Competing forces in the self-assembly of amide-functionalized discotic mesogens. Soft Matter 15(48):10035–10044.
Wheeler, S E . 2013. Understanding Substituent Effects in Noncovalent Interactions Involving Aromatic Rings. Accounts of Chemical Research 46(4):1029–1038.
Geelhaar, Thomas, Griesar, Klaus & Reckmann, Bernd . 2013. 125 Years of Liquid Crystals-A Scientific Revolution in the Home. Angewandte Chemie International Edition 52(34):8798–8809.
Yu, Wen-Hao, Wang, Yan-Hong, Feng, Chun, Ni, Hai-Liang, Hu, Ping, Wang, Bi-Qin, Zhao, Ke-Qing & Fu, Qiang . 2023. Room-temperature triphenylene-based discotic liquid crystal dimers: effects of amide linkage and central fluoromethylene spacer. Liquid Crystals 1–12.
Sutton, Christopher, Risko, Chad & Brédas, Jean-Luc L . 2016. Noncovalent Intermolecular Interactions in Organic Electronic Materials: Implications for the Molecular Packing vs Electronic Properties of Acenes. Chemistry of Materials 28(1):3–16.
Thorley, Karl J, Finn, Tristan W, Jarolimek, Karol, Anthony, John E & Risko, Chad . 2017. Theory-Driven Insight into the Crystal Packing of Trialkylsilylethynyl Pentacenes. Chemistry of Materials29(6):2502–2512.
Kirinda, Viraj C, Vemuri, Gopi Nath, Kress, Nicholas G, Flynn, Kaitlyn M, Kumarage, Nuwanthika Dilrukshi, Schrage, Briana R, Tierney, David L, Ziegler, Christopher J & Hartley, C Scott . 2021. Fluorine Labeling of ortho-Phenylenes to Facilitate Conformational Analysis. The Journal of Organic Chemistry 86(21):15085–15095.
Wöhrle, Tobias, Wurzbach, Iris, Kirres, Jochen, Kostidou, Antonia, Kapernaum, Nadia, Litterscheidt, Juri, Haenle, Johannes Christian, Staffeld, Peter, Baro, Angelika, Giesselmann, Frank & Laschat, Sabine . 2016.Discotic Liquid Crystals. Chemical Reviews 116(3):1139–1241.
Zhang, Zhi, Li, Yunfeng, Zhang, Rentao & Yu, Xiaoming . 2021. Total Synthesis of Geldanamycin. The Journal of Organic Chemistry 86(21):15063–15075.
Zellman, C O & Williams, V E . 2021. Stereochemistry, Conformational Dynamics, and the Stability of Liquid Crystal Phases 86:15076–15084.
Zellman, Carson O & Williams, Vance E . 2021. Stereochemistry, Conformational Dynamics, and the Stability of Liquid Crystal Phases. The Journal of Organic Chemistry 86(21):15076–15084.
Zellman, Carson O & Williams, Vance E . 2022. Semirigid discotic dimers: flexible but not flexible enough? Physical Chemistry Chemical Physics 25(2):1363–1371.
Yeates, T O . 2017. Geometric Principles for Designing Highly Symmetric Self-Assembling Protein Nanomaterials. Annual Review of Biophysics 46(1):23–42.
Sun, Hao-Jan J, Zhang, Shaodong & Percec, Virgil . 2015. From structure to function via complex supramolecular dendrimer systems. Chemical Society Reviews 44(12):3900–3923.
Bates, Frank S & Fredrickson, Glenn H . 1999. Block Copolymers—Designer Soft Materials. Physics Today 52(2):32–38.
Wen, Tao, Ni, Bo, Liu, Yuchu, Zhang, Wei, Guo, Zi-Hao H, Lee, Yi-Chien C, Ho, Rong-Ming M & Cheng, Stephen Z D . 2021. Towards achieving a large-area and defect-free nano-line pattern via controlled self-assembly by sequential annealing. Giant 8:100078.
Qu, Zhiyu, Cheng, Stephen Z D & Zhang, Wen-Bin Bin . 2021. Macromolecular Topology Engineering. Trends in Chemistry 3(5):402–415.
Rohr, Ulrike, Kohl, Christopher, Müllen, Klaus, Van De Craats, Anick & Warman, John . 2001. Liquid crystalline coronene derivatives. Journal of Materials Chemistry 11(7):1789–1799.
Wei, Wei-Shao S, Xia, Yu, Ettinger, Sophie, Yang, Shu & Yodh, A G . 2019. Molecular heterogeneity drives reconfigurable nematic liquid crystal drops. Nature 576(7787):433–436.
Kwok, Man-Hin H, Bohannon, Caleb A, Crooks, Jordan L, Li, Ruipeng, Zhao, Bin & Zhu, Lei . 2020. Grafting density-induced smectic A to hexagonal columnar transition in mesogen-free isotactic liquid crystalline polyethers with n-dodecylsulfonyl side groups. Giant 1:100003.
Zhang, Wei, Dong, Xuehui & Cheng, Stephen Z D . 2019. Reaction: Precision Macromolecules for Self-Assembly. Chem 5(3):492–493.
Shan, Wenpeng, Zhang, Wei, Huang, Mingjun, Ji, Yuyang, Zhang, Ruimeng, Zhang, Rui, Su, Zebin, Liu, Hao, Feng, Xueyan, Guo, Dong, Huang, Jiahao, Liu, Tong, Li, Tao, Mao, Jialin, Wesdemiotis, Chrys, Shi, An-Chang C & Cheng, Stephen Z D . 2020. Fine-tuned order-order phase transitions in giant surfactants via interfacial engineering. Giant 1:100002.
Chen, Yuan & Wu, Shin-Tson T . 2013. Electric field-induced monodomain blue phase liquid crystals. Applied Physics Letters 102(17):171110.
Loman-Cortes, Paula, Jacobs, Donald J & Vivero-Escoto, Juan L . 2021. Molecular dynamic simulation of polyhedral oligomeric silsesquioxane porphyrin molecules: Self-assembly and influence on morphology. Materials Today Communications 29:102815.
Du, Zhen, Fan, Baoer, Dai, Qiuju, Wang, Lan, Guo, Jia, Ye, Zushan, Cui, Naifu, Chen, Jie, Tan, Kun, Li, Ruixin & Tang, Wen . 2022. Supramolecular peptide nanostructures: Self-assembly and biomedical applications. Giant 9(9):100082.
Krishnan, Aravind, Roy, Smitha & Menon, Sajith . 2022. Amphiphilic block copolymers: From synthesis including living polymerization methods to applications in drug delivery. European Polymer Journal172:111224.
Shao, Yue & Yang, Zhenzhong . 2022. Progress in polymer single-chain based hybrid nanoparticles. Progress in Polymer Science 133:101593.
Lei, H, Li, X H, Liu, Y, Liu, X Y, Li, W Y, Yan, X Y, Huang, M, Cheng, S Z D & Huang, J . 2023. Diverse superlattices constructed via perylene bisimide type of giant shape amphiphiles: Assisted with unimolecular nanoparticles: To commemorate Professor Bernhard Wunderlich for his significant and milestone contributions on polymer physics and thermal analysis. Thermochim Acta 719.
Su, Zebin, Zhang, Ruimeng, Yan, Xiao-Yun Y, Guo, Qing-Yun Y, Huang, Jiahao, Shan, Wenpeng, Liu, Yuchu, Liu, Tong, Huang, Mingjun & Cheng, Stephen Z D . 2020. The role of architectural engineering in macromolecular self-assemblies via non-covalent interactions: A molecular LEGO approach. Progress in Polymer Science 103:101230.
Vinayakumara, D R, Ulla, Hidayath, Kumar, Sandeep, Satyanarayan, M N & Adhikari, Airody Vasudeva . 2018. New fluorescent columnar mesogens derived from phenanthrene–cyanopyridone hybrids for OLED applications. Materials Chemistry Frontiers 2(12):2297–2306.
Kim, Youngju, Lee, Mongryong, Wang, Hyuck Sik & Song, Kigook . 2018. The effect of surface polarity of glass on liquid crystal alignment. Liquid Crystals 45(5):757–764.
Shah, Aayush A, Kang, Heekyoung, Kohlstedt, Kevin L, Ahn, Kyung Hyun, Glotzer, Sharon C, Monroe, Charles W & Solomon, Michael J . 2012. Liquid Crystal Order in Colloidal Suspensions of Spheroidal Particles by Direct Current Electric Field Assembly. Small 8(10):1551–1562.
Bala, Indu, De, Joydip, Gupta, Santosh Prasad, Singh, Harpreet, Pandey, Upendra Kumar & Pal, Santanu Kumar . 2020. High hole mobility in room temperature discotic liquid crystalline tetrathienoanthracenes. Chemical Communications 56(42):5629–5632.
Ratto, Carlos, Westphal, Eduard, De Campos, Carlos Eduardo Maduro & Gallardo, Hugo . 2017. Tris(N-phenyltriazole) derivative – New compound with star shaped anisometry and discotic liquid crystals behavior. Molecular Crystals and Liquid Crystals 657(1):147–155.
Schweicher, Guillaume, Garbay, Guillaume, Jouclas, Rémy, Vibert, François, Devaux, Félix & Geerts, Yves H . 2020. Molecular Semiconductors for Logic Operations: Dead?End or Bright Future? Advanced Materials 32(10):1905909.
Fukuda, Kunito & Asakawa, Naoki . 2017. Angular-Dependent EDMR Linewidth for Spin-Dependent Space-Charge-Limited Conduction in a Polycrystalline Pentacene. Frontiers in Materials 4.
Schweicher, Guillaume, Garbay, Guillaume, Jouclas, Rémy, Vibert, François, Devaux, Félix & Geerts, Yves H . 2020. Molecular Semiconductors for Logic Operations: Dead?End or Bright Future? Advanced Materials 32(10):1905909.
Gupta, Ravindra Kumar, Das, Dipjyoti, Gupta, Monika K, Pal, Santanu Kumar, Iyer, Parameswar Krishnan & Achalkumar, Ammathnadu S . 2017. Electroluminescent room temperature columnar liquid crystals based on bay-annulated perylene tetraesters. Journal of Materials Chemistry C 5(7):1767–1781.
Bates, Christopher M & Bates, Frank S . 2017. 50th Anniversary Perspective: Block Polymers—Pure Potential. Macromolecules 50(1):3–22.
Guillamat, Pau, Ignés-Mullol, Jordi & Sagués, Francesc . 2016. Control of active liquid crystals with a magnetic field. Proceedings of the National Academy of Sciences 113(20):5498–5502.
Simonário, P S, De Andrade, T M & Freire, F C M . 2016. Elastic Constants of a Disc-Like Nematic Liquid Crystal: Pseudo-Molecular Approach. Brazilian Journal of Physics 46(1):26–34.
Kristiansen, Kai, Zeng, Hongbo, Zappone, Bruno & Israelachvili, Jacob N . 2015. Simultaneous Measurements of Molecular Forces and Electro-Optical Properties of a Confined 5CB Liquid Crystal Film Using a Surface Forces Apparatus. Langmuir 31(13):3965–3972.
Sahu, Dinesh Kumar, Anjali, Thriveni G, Basavaraj, Madivala G, Aplinc, Jure, ?opar, Simon & Dhara, Surajit . 2019. Orientation, elastic interaction and magnetic response of asymmetric colloids in a nematic liquid crystal. Scientific Reports 9(1).
Kularatne, Ruvini S, Kim, Hyun, Boothby, Jennifer M & Ware, Taylor H . 2017. Liquid crystal elastomer actuators: Synthesis, alignment, and applications. Journal of Polymer Science Part B: Polymer Physics55(5):395–411.
Tschierske, C . 2013. Development of Structural Complexity by Liquid-Crystal Self-assembly. Angewandte Chemie International Edition 52(34):8828–8878.
Jiang, H . 2010. Organic Ambipolar Conjugated Molecules for Electronics: Synthesis and Structure-Property Relationships. Macromolecular Rapid Communications 31(23):2007–2034.
Lin, Hang, Lv, Qiu-Bing B, Wang, Hai-Feng F, Zhao, Ke-Qing Q, Hu, Ping, Wang, Bi-Qin F, Heinrich, Benoît & Donnio, Bertrand . 2022. Organic dyads and triads based on the triphenylene-rylenediimide couple: Molecular design, self-organization, and photo-physical properties. Dyes and Pigments 197:109911.
Jochem, Matthias, Limbach, Daniel, Glang, Stefan, Haspel, Tobias & Detert, Heiner . 2022. Experimental and theoretical investigation on the thermal isomerization reaction of tristriazolotriazines. Journal of Physical Organic Chemistry 35(7).
Westphal, Eduard, Windisch, Alana Carolina, Zambelli Mezalira, Daniela & Gallardo, Hugo . 2022. Reaching Room?Temperature Mesomorphism through Expansion of the Tristriazolotriazine Core with Alkoxybenzoate Units. European Journal of Organic Chemistry 2022(29):2022.
Mu, Bin, Ma, Tianshu, Zhang, Zhelin, Hao, Xiangnan, Wang, Liang, Wang, Jingxia, Yan, Hongxia & Tian, Wei . 2023. Thermo?Induced Bathochromic Emission in Columnar Discotic Liquid Crystals Realized by Intramolecular Planarization. Chemistry – A European Journal .
Dong, Lei, Li, Wen & Li, Wei-Shi . 2011. Construction of a long range p/n heterojunction with a pair of nanometre-wide continuous D/A phases. Nanoscale 3(9):3447.
Basurto, Sara, García, Susana, Neo, Ana G, Torroba, Tomás, Marcos, Carlos F, Miguel, Daniel, Barberá, Joaquín, Ros, M Blanca & De La Fuente, M Rosario . 2005. Indene and Pseudoazulene Discotic Liquid Crystals: A Synthetic and Structural Study. Chemistry - A European Journal 11(18):5362–5376.
Wang, Mingfeng & Wudl, Fred . 2012. Top-down meets bottom-up: organized donor–acceptor heterojunctions for organic solar cells. Journal of Materials Chemistry 22(46):24297.
Rieth, Thorsten, Röder, Nico, Lehmann, Matthias & Detert, Heiner . 2018. Isomerisation of Liquid-Crystalline Tristriazolotriazines. Chemistry - A European Journal 24(1):93–96.
Günes, Serap, Neugebauer, Helmut & Sariciftci, Niyazi Serdar . 2007. Conjugated Polymer-Based Organic Solar Cells. Chemical Reviews 107(4):1324–1338.
Wang, Ling, Zhang, Rui, Huang, Ze, Guo, Shengmei, Yang, Jia-Xiang & Kong, Lin . 2022. A multi-stimuli-responsive tetraphenylethene derivative with high fluorescent emission in solid state. Dyes and Pigments197:109909.
Amharar, Sana & Aydogan, Abdullah . 2022. Highly sensitive and cost-effective fluorescent turn-on sensors based on octamethylcalix[4]pyrrole receptor for the detection of fluoride anion. Dyes and Pigments197:109918.
Detert, H . 2018. Tristriazolotriazines: Luminescent Discotic Liquid Crystals. European Journal of Organic Chemistry 2018(33):4501–4507.
Bhuin, Shouvik, Samanta, Pralok K & Chakravarty, Manab . 2022. Efficient and reversible acidofluorochromic features on a solid platform for reusable security writing: A structure-property relationship study on anthracenyl π-conjugates. Dyes and Pigments 197:109944.
Mali, Kunal S, Adisoejoso, Jinne, Ghijsens, Elke, De Cat, Inge & De Feyter, Steven . 2012. Exploring the Complexity of Supramolecular Interactions for Patterning at the Liquid–Solid Interface. Accounts of Chemical Research 45(8):1309–1320.
Li, Donghui, Deng, Nan, Fu, Yiwei, Guo, Chuanhang, Zhou, Bojun, Wang, Liang, Zhou, Jing, Liu, Dan, Li, Wei, Wang, Kai, Sun, Yanming & Wang, Tao . 2023. Fibrillization of Non?Fullerene Acceptors Enables 19% Efficiency Pseudo?Bulk Heterojunction Organic Solar Cells. Advanced Materials 35(6):2208211.
Wang, Tong, Yang, Xiao-Yu Y, Bi, Peng-Qing Q, Niu, Meng-Si S, Feng, Lin, Liu, Jian-Qiang Q & Hao, Xiao-Tao T . 2019. Effective Exciton Dissociation and Reduced Charge Recombination in Thick?Film Organic Solar Cells via Incorporation of Insulating Polypropylene. Solar RRL 3(8):1900087.
Huang, Jiang, Wang, Hanyu, Yan, Kangrong, Zhang, Xiaohua, Chen, Hongzheng, Li, Chang-Zhi Z & Yu, Junsheng . 2017. Highly Efficient Organic Solar Cells Consisting of Double Bulk Heterojunction Layers. Advanced Materials 29(19):1606729.
Cui, Yong, Xu, Ye, Yao, Huifeng, Bi, Pengqing, Hong, Ling, Zhang, Jianqi, Zu, Yunfei, Zhang, Tao, Qin, Jinzhao, Ren, Junzhen, Chen, Zhihao, He, Chang, Hao, Xiaotao, Wei, Zhixiang & Hou, Jianhui . 2021.Single?Junction Organic Photovoltaic Cell with 19% Efficiency. Advanced Materials 33(41):2102420.
Zhang, Guichuan, Xia, Ruoxi, Chen, Zhen, Xiao, Jingyang, Zhao, Xuenan, Liu, Shiyuan, Yip, Hin-Lap L & Cao, Yong . 2018. Overcoming Space-Charge Effect for Efficient Thick-Film Non-Fullerene Organic Solar Cells. Advanced Energy Materials 8(25):1801609.
Wang, Tong, Niu, Meng-Si S, Guo, Jia-Jia J, Zhang, Kang-Ning N, Wen, Zhen-Chuan C, Liu, Jian-Qiang Q, Qin, Chao-Chao C & Hao, Xiao-Tao T . 2020. 3D Charge Transport Pathway in Organic Solar Cells via Incorporation of Discotic Liquid Crystal Columns. Solar RRL 4(5):2000047.
Van Winkle, Madeline, Scrymgeour, David A, Kaehr, Bryan & Reczek, Joseph J . 2018. Laser Rewritable Dichroics through Reconfigurable Organic Charge?Transfer Liquid Crystals. Advanced Materials30(20):1706787.
Lin, Feng, Yang, Guang, Niu, Chao, Wang, Yanan, Zhu, Zhuan, Luo, Haokun, Dai, Chong, Mayerich, David, Hu, Yandi, Hu, Jonathan, Zhou, Xufeng, Liu, Zhaoping, Wang, Zhiming M & Bao, Jiming . 2018. Planar Alignment of Graphene Sheets by a Rotating Magnetic Field for Full Exploitation of Graphene as a 2D Material. Advanced Functional Materials 28(46):1805255.
Swager, T M . 2022. Molecular Shape and Polar Order in Columnar Liquid Crystals. Accounts of Chemical Research 55(20):3010–3019.
Wöhrle, T, Wurzbach, I, Kirres, J, Kostidou, A, Kapernaum, N, Litterscheidt, J, Haenle, J C, Staffeld, P, Baro, A, Giesselmann, F & Laschat, S . 2016. Discotic Liquid Crystals. Chem Rev 116:1139–1241.
Shen, Jun & Okamoto, Yoshio . 2016. Efficient Separation of Enantiomers Using Stereoregular Chiral Polymers. Chemical Reviews 116(3):1094–1138.
Yampolskii, Yuri & Belov, Nikolay . 2015. Investigation of Polymers by Inverse Gas Chromatography. Macromolecules 48(19):6751–6767.
Zhang, Zhenhu, Yang, Huanzhi, Bi, Jingze, Zhang, Ao, Fang, Yi, Feng, Yuwen, An, Li, Liang, Lijuan, Zhang, Chunxiu & Pu, Jialing . 2018. The leading role of the steric hindrance effect and dipole–dipole interaction in superlattice nanostructures formed via the assembly of discotic liquid crystals. New Journal of Chemistry 42(24):20087–20094.
Bechtold, I H, Eccher, J, Faria, G C, Gallardo, H, Molin, F, Gobo, N R S, De Oliveira, K T & Seggern, H Von . 2012. New columnar Zn-phthalocyanine designed for electronic applications. Journal of Physical Chemistry B 116:13554–13560.
Keywords: Discotic liquid crystals (DLCs),Scanning tunneling microscopy,Optoelectronics,Liquid-solid interface,Nanoparticles self-assembly
Materials Innovations (MI) is an interdisciplinary journal devoted to significant experimental and theoretical findings on the synthesis, structure, charachterization, processing and applications of materials. Materials Innovations is dedicated to publishing peer reviewed novel, cutting edge reports of broad interest to the materials science community.