
Full Html
Vol 3 Issue 5
Advances in Smart Self-cleaning Coatings with Anti-corrosive Properties for Application in Automotive
Pages: 53-64
Doi: 10.54738/MI.2023.3501
Doi URL: http://doi.org/10.54738/MI.2023.3501
1 Institute of Physics, Bahauddin Zakariya Univeristy, Multan, Pakistan
In many technical applications, currently focus is on smart coatings for corrosion prevention. In order to meet the demands of various new high-tech applications, this overview covers the most current advancements in the field of functional coatings which overcome conventional uses. The contributions of smart coating that combine many technologies were highlighted. Significance of functional smart coatings were highlighted because of their importance in both industrial and biomedical applications. Several coating types such as metallic, organic, inorganic, and composite were covered briefly. We also covered many forms of smart coating, their mechanisms, formulations for processing, and various treatment methods. However, conclusion is drawn by considering problems and its prospects for the future, which would be helpful for upcoming researchers. In order to achieve long-term effect in the future, the development direction of smart coating is presented, and potential application possibilities of these smart coatings are examined.
Keywords
Industrial coatings, Organic coatings, Smart coatings
In between 1920 and 1940, alkyd resin-based spray apparatus were introduced in automobile coating technologies. In 1923, E.I. DuPont De Nemours developed nitrocellulose lacquer systems that could be applied with paint spray guns and had a variety of color possibilities. Due to their chemical composition, most lacquer systems have a relatively low resistance to chemical solvents like hydrochloric acid and needed the application of 3–4 layers to attain the necessary surface characteristics. This drawback made it harder for coatings to withstand acidic conditions and other chemicals. The introduction of "alkyd" coating on some automobile of particular models during 1930s was another key breakthrough in paint technology. These enamels produced a coating that was incredibly durable due to bonding processes that happened after when the paint was sprayed onto the cars. While in 1960s advent of acrylic staving enamels, the longevity of enamel coatings was significantly increased 1, 2, 3.
The hand application of the paint spray, however, might result in inconsistent coating thicknesses when the necessary numerous coats were applied. However, different layers were still used at this point for various purposes, such as corrosion protection for the primers, chip resistance and smoothness for the primer surfaces, which were frequently used on the front ends and exposed areas of the4, 5.
A colored enamel basecoat was applied before a clear enamel finish in the topcoat painting procedure. The clearcoat material creation with excellent resilience in all climates was essential to the success of this new technology. The basecoat paint method was utilized on more costly, high-end vehicles even though its cost was prohibitive for the less priced car lines. The cost of materials and processing then decreased, and in 1980s, clearcoat method was widely used. As a result, only a tiny percentage of automobiles produced today do not employ this painting technique. In addition, Opel in Germany launched the first water-based basecoats in the 1980s, followed by water-based primer surfacers in the 1990s. Therefore, in a short amount of time, paint application processes for automobiles had developed to be very efficient and long-lasting, as well as well-matched with the desired requirements of industry. This thickness suggests that estimated (9 -16) kg of paint is used in each automobile. Importantly, it is predicted that today's color and gloss durability and corrosion protection are almost twice as good as they were 25 years ago. The majority of clear coatings used now in Europe are formulations of two-component (2K). This mixture contains a reactive polyurethane crosslinker and an acrylic resin with OH functions. A one-component of acrylic resins and melamine crosslinkers formulation is typically used throughout the remainder of the world 6, 7, 8.
Improvements in processing and paint chemistry have been made with novel advances in paint pigments. For instance, distortion pigments that alter color rely on the viewing angle (often referred by the "flip flopping" affect) and flake-based paints made from aluminum have enhanced the brightness, color, appearance, and consumer satisfaction of automotive coatings. These novel paints were first difficult to employ with technology of spray gun but to address these difficulties, with the combinations of novel spray guns and spray gun have been created. The necessity to provide adequate coating with a uniform thickness regardless of whether these top surfaces were comparatively flat or significantly curved required considerable artistry when spray painting by hand. With the advent of computer-controlled spray guns, the demand for skilled spray painting has significantly decreased. Additionally, advances to these automated processes have increased the proportion of deposited paint to paint sprayed and ensured the safety of the workers. The utmost expensive functioning component of an automotive assembly plant and a significant energy consumer, vehicle paint shops account for 30% to 50% of the overall expenditures associated with producing cars. These expenses are included in the energy used for HVAC, paint drying, and the emissions treatment caused by the pigment droplets which are not left on the surfaces of automobiles. In addition to it, painting booths must be removed to get rid of evaporating solvent, paint particles, and regulated impurities (like VOCs). Therefore, the energy required for only booth drying is substantial. Painting processes often account for up to 70% of the overall energy expenses in assembly facilities. Although it is possible to calculate that the energy required to dry upto 200-m film upon automobile surface is negligible, it is important to keep in mind that paint drying also involves heating the body of the car, the paint, and the dollies and carriers used to transport the car through the painting process. Cathodic electrodeposition, inorganic pretreatments, powder primer surfacers, liquid basis coats, and single- or two-component diluents transparent coatings are all advantageous, automotive painting procedures are more standardized than ever 9, 10, 11.
In North America, powder coatings are currently employed in all primer surface processes at various truck factories, at Chrysler at all of their manufacturing locations, and in all of their label paint shops. The clearcoat technique also makes use of powder coatings in various BMW factories in Europe. This increase in coating applications with powder has been accompanied by a significant change in the materials used to build car bodies. Today's automotive bodywork, which were formerly mostly built of steel, typically contain up to 30% aluminum and high-strength steel. Other lightweight materials are also being used, such as magnesium, polymer composites composed of glass, and thermosets and thermoplastics reinforced with carbon fiber. Automotive coatings are still evolving in order to either fulfill or anticipate meeting customer demands, environmental restrictions, and cost-of-ownership reductions. The use of smart coatings is one of these evolutions since they have the potential to dramatically increase surface strength while introducing new functionality or characteristics like self-stratifying, self-healing, self-sensing, super-hydrophobicity, vibration and sound reducing. In reaction to an abrasive, material trigger event, the smart coating having self-healing qualities would be advantageous. This is because the layer is self-healing as a response of UV, heat, or dynamic activation. For instance, a smart coating may react to it to prolong the coating life. Self-healing may also be accomplished by using polymers that are activated by changes in temperature, humidity, or UV radiation. Additionally, self-healing connected to the swelling of certain clays and the possibility of these montmorillonite exists. Other intelligent coatings come equipped with inbuilt sensors that activate fluorescent molecules or quantum dots in a passive or active manner. Later on, sensing system sends data to an external detector to indicate and trigger changes in or repairs to the coating then the sensing system would be in charge of producing response signal 12, 13, 14.
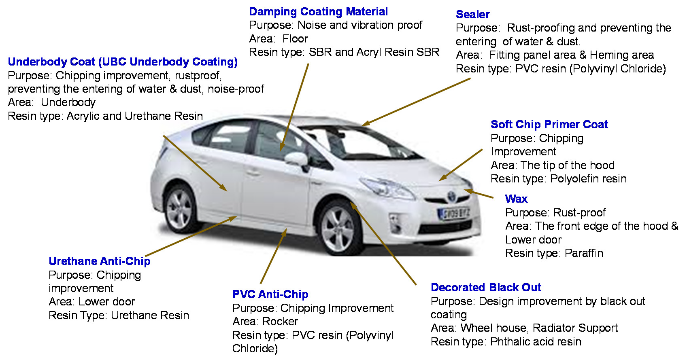
Mainly, there exists five key phases in modern car painting techniques. Among them few of them includes Pretreatment eliminates superfluous metal, cleans it, and creates a surface structure that will allow a corrosion protection layer to adhere. The anti-corrosion layer is electrodeposited (ED). A sealer such as Poly-Vinyl-Chloride (PVC) is used to prevent corrosion, stop water leaks, reduce chipping, and dampen vibration sounds. Next, a primer is used to help the surface and basecoat adhere to one another. It also provides the flatter surface for following coats and possesses anti-chipping qualities. Figure 1 shows the regions of such an automobile in which these five coating stages as well as other paints are applied. The necessity for just a substantial number of particular coatings and substances, in addition here to stages mentioned above, is evident when looking at about this figure.
Pretreatment is the initial step in formulating the BIW following coating as shown in Figure 2. The three basic liquid dip techniques for pretreatment include washing the body surface to get rid of any leftover lubricants from the stamping process and welding residues. It is also possible to do a further preparatory washing using hot water with a pH of 9. The primer will adhere better to the metal after pretreatment. An inert coating of metal phosphate is applied during phosphate treatment to provide resistance to the propagation of corrosion. The following process, surface conditioning (also known as activation), produces the nucleation sites needed for the growth of phosphate crystals. It raises the proportion of crystallization upon the metal face, which improves the bonding processes for the next phosphate sequence. Conditionally, titanium orthophosphate in an aqueous dispersion with a pH of 8 to 10 is utilized. The dip phosphate solution is made up of phosphoric acid, nitrate ions, phosphate ions, zinc and other bivalent metal ions, hydrogen ions, and a chemical. A crystalline form of ions of metal phosphate precipitates onto the steel surface as a result of the free acid's erosive action, which releases hydrogen while etching the steel surface. The main goal of the phosphating process is deposition of a thin, thick, and uniform layer on the cleaned and ready metal surface 16, 17, 18.
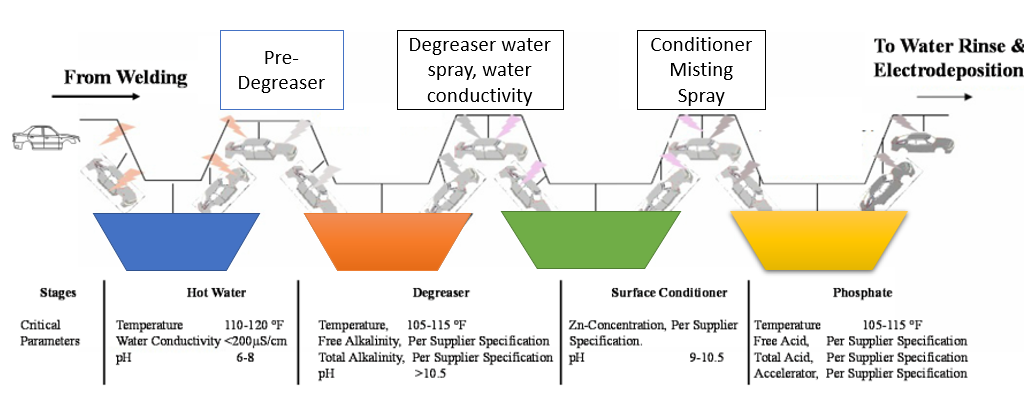
Figure 2: Sequences of Pretreatment for a body-in-white. Reproduced with permission from ref 15. Copyright 2016 MDPI
The basic coating for preventing corrosion in contemporary automobiles is a SCGA deposition solution with a composition of 90% Zn and 10% Fe (GA material). While the SGC procedure is still employed in Europe, The GA substance enhances spot welding capability while minimizing ED gas pin (paint texture flaws). When compared to the SGC solution, or 100% Zn-containing GI material, at electrodeposition.19 It was possible to minimize the use of ED gas pin when the SCGM corrosion prevention technology was being used in earlier decades. Contrary to the GA material covering, it was more expensive. It was initially announced in 1960s, and the electrodeposition coat, often known as the E-coat, protects against rust and corrosion. Since that time the E-coat usage has rapidly increased. By 1970, 10% of all automobiles had electrocoating, and by 1990, 90% of all automobiles had electrocoating. It is currently the most common coating technique used in automobile manufacturing. During this time, the E-coat film's thickness has also changed 20, 21 22.
E-coating includes dipping automotive bodywork in the coating solution as depicted in Figure-3 and then applying the electric current to the body whereas liquid ED paint solution is still in contact with the body. The ED paint may penetrate into spaces that a spray paint would not be able to due to the charged nature of this coating procedure. It is encouraged for the ED paint to adhere at metal substrate, and this results in a homogeneous paint thickness. The end product is an insoluble, deposited layer that is firmly adhered 23.
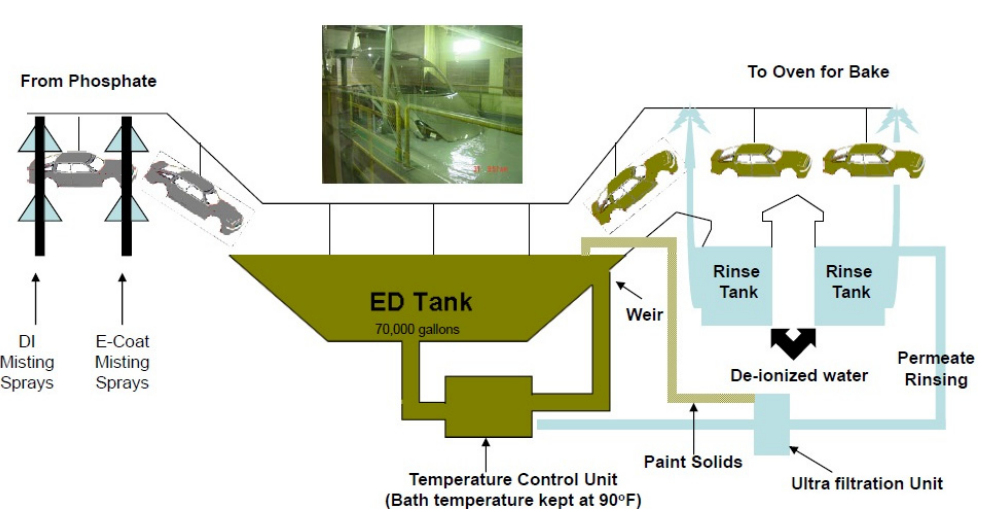
Figure 3: Electrocoating process. Adopted from ref 15
During use, the electrodeposition tank receives a feed of binder, resin, and a paste pigments. The tank contains a solution of 80%–90% deionized water and 10%–20% paint solids. The car body is lowered into the tank, and an electric current is applied (containing the pigment, resin, and binder). The constantly agitated paint solids are transported by the deionized water. The resin, which provides corrosion protection, toughness, and longevity, forms the framework of the finished paint film. Color and gloss are produced by pigments. The covering gets more insulating as it thickens. The deposition process slows down as the covering becomes thicker and more insulating. In order to provide a smooth surface as a car body leaves the tank, paint solids that are advocate to the surface but not fixed to it are washed off and recovered as ED solids using an ultrafiltration machine. 24
In recent years, PVC and the acryl/urethane sealants have also been employed in the underbody regions, a procedure known as the Reducing Coat (DC), to provide the noise-proofing and the vibration-deadening. The underbody sealants minimize noise transfer into the car's passenger area by absorbing sounds coming from the tires, road, engine, drive train, suspension, and moving air. A robot fitted with an airless sprayer is commonly used to apply in underbody painting, which resist against corrosion and produced chipping protection. In Figure 6, the door below segments and rocker sheet places where PVC or the urethanes are placed for anti-chipping protection are shown, together with the cross section of this coating layer to relate it with some other coating layers on the body surface parts 25, 26, 27.
A smooth tip primer paint that increases the resistance to chipping is typically applied in the third phase as well. A more elastic resin which is sandwiched between ED and the primer coats is used to apply this layer at anterior hood edge, which is disposed to to chipping shock. The third process also involves applying a blackout coating, a somewhat flat black pigment, to supports the radiator, wheel housing, and underneath of the body. Primer surface, often known as primer, is applied during the fourth coating process. It could come in the form of a powder, a solvent, or water. Prior to 1990, improving weather resistance, aesthetics, and chip resistance were the key reasons for using primer. Since 1990, the development of primers for the use of solute, water-borne, and powdered particles explicitly decrease the quantity of organic pollutants (VOCs) released into the environment. Today's primers need to be more ecologically friendly and compliant with emission requirements, as well as better adhesion between E-coat and the topcoat, offer protection against chipping, improve paint look, and more. 28, 29, 30 19
Applying the topcoat, which is composed of the basecoat and clearcoat in two coats, is the last stage in the body coating procedure. The basecoat is the key coloring pigment, also clearcoat offers protection from the elements, corrosion, and the UV light deterioration, indorses unparalleled color retaining, and offers a smooth, flawless, and uniform finish. A water-borne basecoat is applied to the body initially. For wet-on-wet manufacturing method, the clearcoat has put over the basecoat following a brief flash-off and is then baked to cure. Usually, basecoat is applied first and then clearcoat is employed, when a wet-on-wet method is not used. The normal thermal cure times and temperatures, regardless of the technique used, are 30 to 40 min at 125 °C. These final coats are applied in spray booths equipped with paint and VOC capture machinery as well as air management systems for controlling temperature, humidity, and cleanliness. Filtration media for controlling dust and debris are included in the air handling system. Paint particles are captured in wet scrubbers using water and strong airflow. Paint splatters are carried into the collecting apparatus in the booth's underside in order to control air flow inside the booth.31 Evolution of different methods of the automotive painting process is shown in Figure 4.
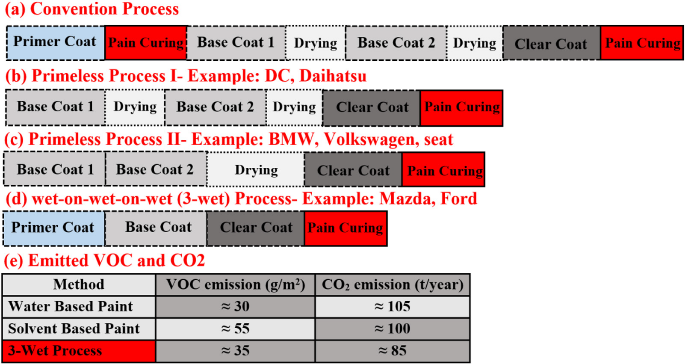
Figure 4: Evolution of different methods of the automotive painting process, Adapted from Re 32, Copyright 2020 Author(s), licensed under a Creative Commons Attribution (CC BY) License.
Smart coatings are created ingeniously for a range of applications and have the ability to respond to stimuli in an unpredictable way. This innovative idea has sparked intriguing research in this direction to ascertain their utilities. Anticorrosive coatings now have much value thanks to the multifunctional characteristics of smart coatings that have been used to prolong the metallic substrates equipment life. Smart coatings react to the aggressive changes brought on by altering the surface tension, pH, temperature, ionic strength, pressure, noises, light, electrical or the magnetic fields, mechanical factors, including abrasions, etc., leading to particular photocatalytic, acid-base, complication, bond forming, electrochemical processes, etc. As other additional features are sought after, the bulk of these coatings are developed in interaction to the inhibitors to ensure that anti-corrosive characteristics are improved. Reliant on the thickness, mixture, and specific practical purpose, different corrosion inhibitor-loaded coatings are applied. 33, 34, 35
Internal stimuli are signals that cause changes to coatings' bulk characteristics and cause them to react. Self-healing and corrosion-sensing coatings exhibit this. External stimuli, on the other hand, come from signals whose reactions change how a coating's surface properties relate to its surroundings, as observed as self-cleaning and the anti-fogging coatings. The performances are improved when dynamic and inactive components are incorporated to smart coatings. This offers quick responses to changes, such as abrasions and coating cracks, as well as variations in the temperature, the pH, and the salinity. Due to intricate makeup of the current coating-metallic substrate schemes, it has been discovered that some coatings' high-performance levels decrease when they are put to use. The target coating durability and attainment depend on the kind of substrate, coating thickness, substrate pretreatment, the curing time, ambient conditions, and coating adhesion to the substrate. 36, 37, 38
In order to find oxygen reduction after the process of oxidative corrosion, these coatings must typically be pH sensitive. The metals and the alloys painted with such undergo an elevated pH at the location before corrosion starts to occur. These coatings possess color-varying chemicals and/or dyes that glow or change color as a result of oxidation for the higher pH values or they form complex compound with the metal cations in the event of possible mechanical damage. The majority of the matrices used are transparent so that when the corroding species reacts with them, the color change or fluorescence is evident. Additionally, these pigments are planned to release anticorrosive agents upon injury or when corrosive species are detected without causing visible color changes. The creation of the coatings and the paints made of polymeric materials has benefited from advancements in this field. Inhibiting additives and primer coatings have been combined with substances including Schiff bases, the hydroxyquinolines, the fluorescein, the phenolphthalein, the oxines, the bromothymol blue, the 7-amino-4-methylcoumarin, and others for this purpose. The majority of fluorescent systems is categorized as active, while color-changing fluorescent systems are considered passive. [39-42]39, 40, 41 42
A surprised feature of corrosion inhibitor compositions is the capacity of coatings to fully recover and exclusive to the "smart" segment of surface coatings as a result that there is a significant delay in corrosion while they are in use and there is consequent fall upon final occurrence. By collection development inhibiting compounds into already-existing polymer-based coatings, self-healing coatings are created. Their mechanism includes the slow release of film constituting inhibitors from the partially collapsed coating composite to provide a protective cover build up via adsorption on the open surface of the metal or an alloy. By definition, such types of coatings are guarantee of the polymer matrix is restored in a way that preserves its mechanical qualities and prevents onset corrosion. The chemical structure and functional properties for self-healing coating, which is based on the biological wound healing process, determine how well it works. Once most relevant applications of the structural self-healing is the rusting protection, whereas the active elements are the inhibitors with reacting groups like the free radicals, the aromatics, [SiO, CC, COOH, NH2, SH, SSe], etc., either in micro or nano-forms. [124] It's interesting that study and interest have recently focused on healing coatings or self-repair coatings. Microparticles and nanoparticles have reportedly been added to polymeric substances to achieve the desired results, including durability, ease of application, cost effectiveness, repeatability, improved the surface morphology, and the eco-friendliness. For these acknowledged characteristics, they serve as inhibitor constituent part in the polymer coatings that contain additional inhibitors. Intrinsic and extrinsic influences of the self-healing phenomenon have been distinguished. In the latter, healing chemicals incorporated in the polymer covering are thought to work once a trigger is applied. Overview of main loading and release methods of corrosion inhibitors is shown in Figure 5 43, 44.
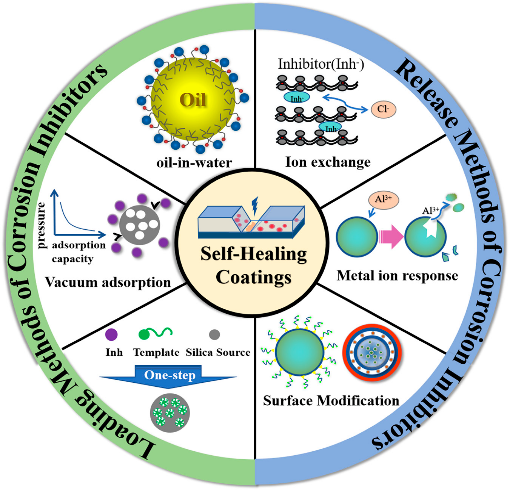
Figure 5: Overview of main loading and release methods of corrosion inhibitors Reproduced with permission from ref 44. Copyright Frontiers in Materials 2021
The following factors have been used to explain the phases of self-healing preservations of cracks and tiny defects. Surface rearrangement: Surface rearrangements exist in cracked polymer matrix surfaces that influence the form and rate of crack healing. The diffusion dynamics can occasionally be complicated by further damage, such as the crack spreading further as a result of oxidation and cross-linking reactions. When the compatible healing ingredient contacts with the polymer's surface. Surface strategy: In this stage, the healing interface formation approach factor for a time-dependent interaction of the various surface components is a concern. Therefore, the presence of fracture development debris may obstruct surface interactions and prevent healing. Additionally, no healing happens if the healing agent exclusively attaches to one surface. Wetting: The approaching surfaces must be wetted in order for them to be compatible, establish an interface, and heal afterwards, especially when using self-repair fluids. At the interface, this likewise happens as time-rely manner. Diffusion and the randomization: The forte development for the self-repair crack healing systems that occur at the polymer-polymer edges depends largely on the diffusion stage. As in welding procedures, the diffusing chains flow and penetrate the fully entangled matrix chains. The connection seems entirely healed regardless of the degree of diffusion when stress is increased and random bond breakage in network takes precedence over the deformation and for disentanglement. 45, 46, 47, 48
Recent advancements in the self-healing coatings using microcapsules comprise those with two-layered microcapsules that use hexamethylene diisocyanate as their primary core substance. From microcapsules to nano capsules, and from the bulk inhibitors and additives to the nano-inhibitors and additives delivering much efficient and numerous effects and self-healing effect has undergone a progressive metamorphosis. 49
These coatings make sure that dirt is autonomously detached from the surface via either through hydrophilic or through hydrophobic method. The surface interaction angle between the liquid drop surfaces of the solid substance is the basis for the self-cleaning experience. In general, a surface is described as hydrophilic if its contact angle is less than 90 degrees, hydrophobic if it is greater than 90 degrees, and super (ultra) hydrophobic if it is greater than 150 degrees. In order to apply these coatings effectively, it is important to pay attention to how water behaves on surfaces and to prevent creating any surface blemishes or fissures that could cause the coating to come off once water reaches them. Natural anti-corrosive self-cleaning coatings that are hydrophobic or hydrophilic in nature clean surfaces. Their intelligence enables them to react to outside elements like the electric field, temperature, light, etc. Wettability and water contact angle are influenced by the solid coating surface's geometric structure and chemical makeup. Additionally, both surface energy and roughness have a significant impact. As a result, air becomes trapped in the surface valleys of a coated substrate with the superhydrophobic surface when it is submerged in hostile solutions. Through a photocatalytic cleaning procedure, hydrophilic coatings are very wettable. This type of coating needs polymers with superior film-forming abilities, as well as flexibility, toughness, and other qualities, such that a modest amount is required to give coatings with increased mechanical attributes including durability and optical transparency. 50, 51, 52, 53
Additionally, there are self-cleaning paints that are both biocidal and self-cleaning, and this is due to the incorporation of nano-silver and nano-titanium oxide into the paint layer. The development of biocidal coating systems with the biocides incorporated in nanoparticles and designed to only release the biocides when necessary. As a result, the biocidal coating has a longer lifespan of activity. Furthermore, composite coatings with low surface energy have been described as self-cleaning for glass surfaces. By adding nanoparticles, transparent coatings were produced that did not interfere with light transmission through the glass. Recent developments in self-cleaning surfaces have also been made possible by micro-nano surfaces, which feature a micro-texture with embedded nanopores and produce a mechanically resilient surface with superamphiphobic qualities 54, 55.
The most popular method for preventing corrosion in metallic items is still organic coatings. Despite their fantastic anticorrosive qualities, they required to be upgraded with more environmentally benign technologies. Therefore, it is necessary to create and apply clever, environmentally friendly organic coatings in more economical and efficient ways in order to lessen corrosion. The functionality these coatings provide at the interface of metal-solution under challenging conditions has aided in the advancement of anticorrosion research and applications. Smart coatings can repair coating faults, respond swiftly to environmental changes, and stop further corrosion. Compared to the conventional anticorrosive coatings, they have better anticorrosion capability. Additionally, it discusses a few different types of waterborne and biobased polyurethanes and hyper branched polyesters, two categories of smart organic coatings with anticorrosive properties 56.
Lord Rayleigh (John Strutt) first noted that tarnishing on glass increased transmission rather than decreased in the 19th century, which gave rise to the antireflective coatings’ idea in physical sciences. When Fraunhofer observed that reflection was lessened as a result of etching a surface in an environment of sulphur and nitric acid vapours, Numerous studies into antireflective coatings have been spurred by the desire to increase light collecting efficiency in order to meet the rising demand for optoelectronic equipment in variety of applications [98]. The performance of optical constituents made from glass-based optical ingredients is improved, and reflecting losses at interfaces are decreased, thanks to antireflection coatings. Consequently, flat-panel displays' antireflective coatings. 57, 58, 59, 60
Phase split polyelectrolyte multilayer coating that go through a reversible pH-triggered swelling transition can be used to create high-efficiency antireflection coatings [99]. Similar to this, a double-layer coating founded on a wavelength system with an index gradient of 4–4 has been described. A sufficient broadband antireflective performance was achieved when coated on the substrate of glass to meet the requirements of the amplifier blast-shields applied for power laser systems. The NH3-heat treatment and trimethylchlorosilane (TMCS) post-treatment were used to address the coating's poor mechanical property and optical instability [100]. Manca et al. created the antireflective surfaces using double-layer coating made up of modified nanosilica particles. 60, 61, 62
The smart coatings are used in variety of End-user applications including automotive medical and healthcare, aerospace, military, aerospace,, maritime, consumer electronics, energy, construction, oil and gas, packaging, textile and clothing sectors, etc. Smart coatings can be modified to match the required of the individual consumers. Self-cleaning Household surfaces can employ thin nanofilms. They shield the surfaces from grease or dust buildup, corrosion assault and scale buildup and are suitable for skin contact. These self-cleaning surfaces, which include kitchen countertops, shower screens, and computer equipment, will reduce the need for detergents and cleaning time. Electronic display screens, Optics, cafés, restaurants, kitchens, floor sealants, textiles, automobiles, HVAC (heating, ventilation, and air conditioning) systems, and other areas have used anti-finger print coatings. When applied to an aircraft's fuselage, the coating fixes minor damage brought on by mechanical and environmental stresses experienced by the aircraft's appliances during flight by releasing its embedded healing molecules as needed. Nissan has also tested Ultra-Ever Dry, an innovative nanopaint technology that is a contribution to the creation of the first self-cleaning car in the world. It minimises the need for frequent car washes by repelling mud, rain, oil, sleet, filth, and ordinary road spray. Although there are currently no plans to add self-cleaning paint as a standard feature to Nissan's car portfolio, the technology is taken into consideration as a prospective aftermarket option. 63, 64 Table 1 represents the resins, the properties and their applications.
Table 1: Represents the Resins, the Properties and their Applications. 65, 66, 67
|
Resin |
Polymer |
Properties |
Applications |
|
Vinyls |
Poly (vinyl acetate) (PVAc) |
Hydrophobic Low adhesion to variety of substrates Recyclable |
Decontamination, Pressure-sensitive peelable adhesive film |
|
Poly (vinyl alcohol) (PVA) |
Water-soluble Non-toxic, Non carcinogenic Biodegradable |
Hydrogels for decontamination, Antimicrobial Food Coatings |
|
|
Poly (vinyl butyral) (PVB) |
Good adhesive strength Toughness Flexible, Clear films |
Protection of Painted Laquer finished surfaces |
|
|
Ethylene-vinyl acetate copolymer (EVA) |
Excellent abrasion resistance Humidity resistance Flexibility |
Opaque backing for the photographic film Corrosion Resistant coatings |
|
|
Poly(vinyl-chloride) and Poly (vinylidene-chloride) (PVC PVDC) |
Water proof, Low moisture permeability Heat resistant |
Corrosion-Protection Water-proof coatings |
|
|
Rewettable coatings |
|||
|
Non hazy clear films |
|||
|
Acrylics |
|
Durability and excellent adhesion with good peelability over a wide temperature range, Hardness Scratch resistance |
Vehicle coating, Construction element coating, Electrical Insulation and Protection of sensitive Electronics |
|
Polyurethanes |
|
Toughness, Abrasion resistance flexibility High elongation, tensile strength |
Floor-Coating, Automobile-coating, Ventilation fan coatings |
|
Cellulosics |
Cellullose Ether |
Water soluble , Non toxic, Non reacting |
Food-grade coatings |
|
Nitrocellulose |
Transparent, Tough film, Low cost |
Cleaning, Medical, Optical Applications Nail Varnish |
The most current developments in functional coatings used to protect metallic substrates against corrosion are reviewed in this article. It is possible to incorporate a range of functions into coatings by encapsulating functional substances in stimuli-sensitive inorganic or polymeric carriers, leading to the development of a new generation of intelligent, responsive materials. Combinatorial healing abilities and multifunctional qualities are made possible by the blending of carriers that are responsive to various stimuli and loaded with healing or functional species with various kinetics of release. There is a lot of interest in self-healing coatings centered on the usage of carriers of healing agent. The usage of hollow particles, capsules, mesoporous particles, tubular reservoirs or layered clays are just a few of the options that have been suggested. Many theories have been tested in the lab, but only a select number have stood up to the rigorous corrosion testing needed for industrial approval. Designing systems with varied healing capabilities, estimating the lifespan of the disseminated or encapsulated healing agents, comprehending the healing kinetics at various ages, and stopping early, uncontrolled carriers leaching are open problems and scientific difficulties. The capacity to repair many damage occurrences must now be modelled, predicted, and validated, and the thresholds for the geometry and size of the repairs must be established. It is well known that smart carriers should contain anti-fouling species, especially if they are paired with species that hinder corrosion. 68 69
To reduce corrosion issues in assets exposed to maritime environments, these characteristics must be combined. The relevance of coatings with functionalities aimed at repelling water, such as hydrophobicity, superhydrophobicity, and ice-repellence is paramount, and the number of innovative products is rapidly increasing. Finding innovative autonomous solutions that combine many capabilities, such as super hydrophobicity, bio-fouling, reduced wear, ice-repellence, drag reduction, and corrosion protection, is a problem in this field. Poly siloxane chemistry's adaptability and flexibility enable the creation of corrosion protection coatings that meet the most recent environmental friendliness standards. Other intriguing surface capabilities are also being studied, for example aqueous soft touch coatings, that are very desirable in market for portable devices as well as the for the interiors of vehicles and airplanes. Additionally, such coatings must offer improved resistance to scratching and abrasion. The fabrication of improved functional coatings for bioresorbable implants is greatly hampered by the biomedical industry's unique needs. Most of such coatings for bioresorbable applications that have been suggested so far have substantial inorganic barrier layers that stall the spread of corrosion. Since polymeric coatings offer appropriate biointerfaces that may be customised for various activities, they are more adaptable alternatives. However, not all of the capabilities that can be added to these coatings have been extensively investigated at once. Nanoparticles can be included into biodegradable organic coatings to promote bone development, cell adhesion, and antibacterial activity. While offering regulated corrosion protection, these coatings can be employed for the controlled distribution of substances like antibiotics or anti-inflammatories. The use of bioresorbable metallic implants will be hampered by such cutting-edge functional coatings. 62, 63, 64, 68, 70
Numerous attempts have been made to encourage actual commercialization to the most appealing technology, which is based on smart coatings. In this study, the majority of coating-related topics have been addressed, including coating types and materials including coating characterizations as well. We discussed smart coatings and their many forms, including self-healing coatings, corrosion-sensing coatings, self-cleaning coatings and anti-wear coatings were also covered in this part. Examples of external influences in automotive includes surface damage caused by corrosion, impact, scuffing, staining, pitting and, rail dust, chemicals, dirt, acid rain, oil, and grease during assembly, manufacture, and movement of car parts from one place to another. These coatings can be used to clean delicate surfaces and shield them from outside forces that might harm them. In many situations, scrubbing and washing with soap are insufficient to get rid of the pollutants and scrubbing is not sufficient to remove contaminants. Various techniques have been used to solve these issues. These days to accomplish this goal. Peel-able coatings are tested for covering construction items that need heavy-duty performance in addition to automobiles. In the past, permanent paint was put to cars to improve appearance, but the procedure is highly expensive and irreversible unless the substrate is repainted. Consequently, a temporary peel-able coating that offers the appropriate look and finishes and can be removed when no longer needed is required.
Keywords: Industrial coatings,Organic coatings,Smart coatings
Materials Innovations (MI) is an interdisciplinary journal devoted to significant experimental and theoretical findings on the synthesis, structure, charachterization, processing and applications of materials. Materials Innovations is dedicated to publishing peer reviewed novel, cutting edge reports of broad interest to the materials science community.