
Full Html
Vol 3 Issue 6
Phyto-Functionalization of MoO3-ZnMoO4 Composite for the Catalytic Wet Oxidation of Methyl Orange Under Dark Ambient Conditions
Pages: 65-73
Doi: 10.54738/MI.2023.3601
Doi URL: http://doi.org/10.54738/MI.2023.3601
Irum Shaheen?? 1,2 , Khuram Shahzad Ahmad?? 1 , Sadia Iram 3
1 Department of Environmental Sciences, Fatima Jinnah Women University, Rawalpindi, Pakistan
2 SUNUM Nanotechnology Research and Application Center, Sabanci University, Tuzla, Istanbul, 34956, Turkey
3 School of Natural Sciences, National University of Sciences and Technology Islamabad 44000, Pakistan
Photocatalysis has gained huge research interest for the degradation of textile dyes from the water environment. In addition, researchers are eager to develop catalysts for the fast degradation of organic dyes in ambient conditions without any stimulants. In this regard, a phytogenic metal oxide-based MoO3/ZnMoO4 catalyst is synthesized in the current study using Abies pindrow Royle (A.pindrow) foliar extract for the degradation of methyl orange from water bodies. A.pindrow mediated MoO3/ZnMoO4 composite is prepared via sol-gel synthesis route following thermal decomposition in air. The phase analysis and chemical composition of the prepared composite are confirmed by powdered X-ray diffraction, energy dispersive spectroscopy, Raman spectroscopy while the surface morphology is examined by field emission scanning electron microscopy. The synthesized MoO3/ZnMoO4 catalyst is employed to degrade MO in the aqueous environment in the presence of solar light and in dark ambient conditions without any stimulation. It is revealed that A.pindrow framework-derived MoO3/ZnMoO4 catalyst exhibits excellent catalytic potential to degrade MO in aqueous solutions with 90% and 99 % degradation efficiency under dark and light conditions, respectively, within 10 minutes. Moreover, the catalyst demonstrates substantial stability until four cycles of experiments with pseudo-first-order kinetics under light and dark conditions (R2 <1). Thus, the overall findings of the present study highly suggest the significant potential of MoO3/ZnMoO4 catalyst for the degradation of MO even in dark ambient conditions.
Keywords
Phytotemplate, Metal Oxides, Methyl Orange, Catalysis, Degradation, Dark conditions
Organic pollutants from the textile, painting, leather, and photography industries are the main environmental hazards to humans, animals, and even microorganisms because the industries and chemical factories release the organic pollutants into water bodies resulting in inadequate access to clean water. Organic environmental pollutants have become the most persistent1-7 Therefore, concerns about the treatment of organic pollutants have widely emerged as a national and international priority in the 21st century. The azo dye is the largest group of synthetic organic colorants, constituting 60 to 70% of all dyes.8-13 The azo dye such as methyl orange (MO) is prominently used in paper, textile, cosmetics, additives, and foods industries for several applications. The MO dyes are extremely harmful, potentially carcinogenic, and most persistent organic compounds, therefore, there is an urgent need to treat and remove MO azo dyes from water bodies.7-18
The various approaches for removing and treating azo dyes have been reported in the literature.5-20 Generally, the methods and techniques involve in dye treatment are adsorption, sedimentation, coagulation, and ion flotation.8-10 Among them, Advanced Oxidation Processes (AOPs) are the comparative most sustainable approach which involves an oxidizing species like •OH radicals to initiate a chain of reactions to break down the organic macromolecules (in most cases) into the water, oxygen and/or carbon dioxide i.e., completely mineralization of the dye. The different techniques in the AOP approach are the photocatalytic approach, Fenton process and photo-Fenton process, the degradation, the ozonation process, and the photo-degradation of dyes.10-15 The degradation of dye by suitable catalysts has drawn extensive attention in the scientific community as it is an ecofriendly, economic AOP technique and produces considerably less residual material compared to the other treatment methods. Thus, catalytic degradation of azo dyes has become a promising tool for water remediation.13-16
Catalysis involves a redox mechanism that decomposes an organic molecule, so the material used as a catalyst should have a high redox potential.14-17 In this regard, semiconducting metal oxides such as ZnO,2-4 Co3O4,15 Fe2O3,4, 10, 15 TiO2 , 11 and V2O5 1 0 have been widely reported in the literature to treat/degrade coloring dyes due to their eco-friendly benefits of saving resources such as energy, water, and other costly chemical cleaning materials. Metal oxides are highly suitable catalytic materials because of their numerous merits, such as low cost, optical-electronic characteristics, chemical stability, and non-toxicity.10-15 It is well reported that metal oxides, with the assistance of solar light or any stimulants, can completely remove the organic pollutants from water bodies.16-20
However, the fast degradation of organic pollutants in the presence of solar light and under ambient conditions without the help of any stimulants is still challenging among the scientific community. Moreover, cost-effective and efficient catalytic materials are highly desired for economical, quick, and direct remediation of azo dyes from water bodies. Consequently, the present study is an attempt to overcome the above-mentioned challenges by synthesis Zn-Mo oxides based catalytic material using phytochemicals of the Abies pindrow Royle (PEAP) leaves as stabilizing and reducing agents. Although some previous reports also utilized plant-assisted preparation of nanoparticles for degradation of organic pollutant,20-25 however, the phyto-synthesis of MoO3/ZnMoO4 using PEAP plant leaves has been carried out for the first time in the current study. PEAP plant leaves were chosen for this purpose because of their richness in multiple PCs like terpenoids, flavonoids, glycosides, phenolic acid as well as flavonol.26-28 These phyto-organic functional groups of PEAP leaves were brought together with MoO3/ZnMoO4 composite as stabilizing agents for degrading MO with and without solar light. Moreover, the current study is the first comprehensive study reporting the maximum degradation (90%) of MO using phytogenic MoO3/ZnMoO4 without any stimulant. We believe that plant-assisted preparation incorporates phyto organic species in prepared catalytic material and thereby enhances its catalytic performance for the degradation of organic dyes.
Molybdenum(II)acetate(Mo?(O?CCH?)?) [95.99%], Zinc acetate dihydrate(Zn(O2CCH3)2 2H2O2) [98%], ethanol [99%] and methanol [99%] were bought from Merck chemicals Ltd. PEAP plant leaves were used as reducing agent as well as stabilizing agents in preparation of MoO3/ZnMoO4 oxide.
In the current study, reported phytosynthesis methodology 23,24 was adopted with modifications.
1.2 grams (0.005 moles) of Zn(O2CCH3)2 (H2O)2 , 1.2 grams (0.0028 moles) of Mo?(O?CCH?)? as well as 2 grams of desiccated ground PEAP leaves were separately added in 100 mL of DI water and each solution was magnetically stirred for almost 30 minutes. PEAP leaves were heated at 60 oC to extract its organic constituents, whereas the precursors solutions were prepared at room temperature (RT). Both precursors solutions were mixed after 30 minutes, whereas PEAP solution was first cooled to RT, filtered and then 20 mL of filtrate was added to the mixed reaction solution under vigorous stirring. The temperature was raised to 70 oC for about 2 h after that undergone incubation at RT under dark conditions for 24 hrs for completion of the phyto-functionalization and hydrolysis process. The resulting solution was then evaporated at 95 oC overnight and was annealed at 450 oC for about 4 hrs to get A.pindrow functionalized Zn-Mo oxides labeled and hereafter written as AP-ZMO.
The catalytic performance of AP-ZMO composite was investigated by the degradation of MO aqueous solution under sunlight (SLMO) and without sunlight in dark ambient (DAMO) reaction conditions. The synthesized AP-ZMO (2 mg) was added into the test tube labeled as SLMO. The same amount of AP-ZMO was added into another test tube labeled as DAMO which was entirely wrapped in Aluminum foil to avoid interference with any stimulants. An aliquot of 10 mL of stock solution of MO was added to the two further different test tubes without catalysts as controlled samples with SLMO and DAMO experiments. All reaction tubes were stirred in the dark for 0.5. After that, SLMO and its controlled sample were placed under solar radiation without the aluminum wrap, while DAMO its blank sample was placed at room condition with an aluminum warp. All samples were then analyzed by ultraviolet, and visible spectroscopy at a range of 200-600 nm with carefully monitoring absorbance at 464 nm. Each sample was tested at regular intervals from 0-15 minutes to determine the percentage of degradation of MO based on absorbance at 464 nm wavelength. The percentage degradation was calculated using the following equation (1)23
1 ??????. (%)= ??-??/?? ×100
In which, Co being initial and Ct is the final concentration of MO.
The optical absorbance of the catalyst was examined by ultraviolet, visible spectroscopy (UV.Vis.) 1602, biomedical services, Spain. The presence of phytochemicals were identified by gas chromatography-mass spectroscopy-GC-MS-QP5050 (SHIMADZU) along with Fourier transforms infrared spectroscopy (FTIR) 8400, Shimadzu, Japan. The other material analysis techniques used in the current study were XRD5 PANaytical X’Pert Pro, Quanta 250-FEG scanning electron microscope (FE-SEM) and Raman spectroscopy (Renishaw System 1000, laser wavelength 514) at the School of Materials, The University of Manchester, U.K.
The PEAP leaves extract reacted with precursor salt solution to incorporate the carbon-oxygen-based phyto organic compounds as stabilizing agents. Thus, phytogenic AP-ZMO has initially been screened for the presence of phytogenic organic species by FTIT and GCMS. FTIR of AP-ZMO has shown in figure 1a revealing the peak frequencies at 864.4 and 734.45 cm-1 relating to the carbon-based organic groups. Inserts present in figure 1(a-) showed M-O bonds present in metal oxides (M=Zn, Mo) within the frequency range 400-500 cm-1.29,30 The peak frequencies revealed in figure 1a FTIR validated the incorporation of phyto phenol along with oxides of metal.
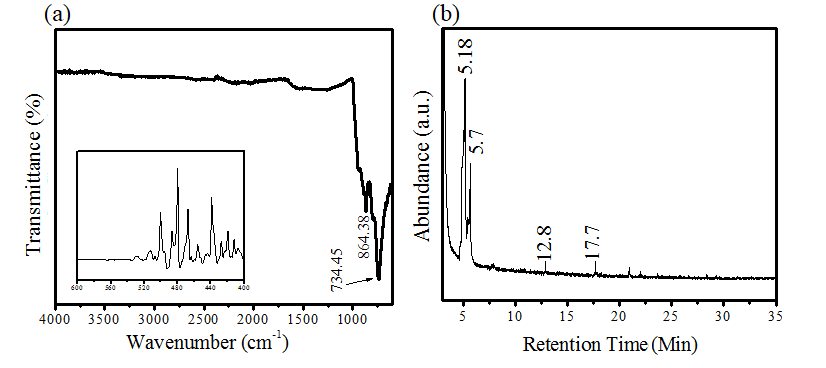
Figure 1: (a) FTIR analysis of AP-ZMO (b) GC-MS chromatogram of AP-ZMO.
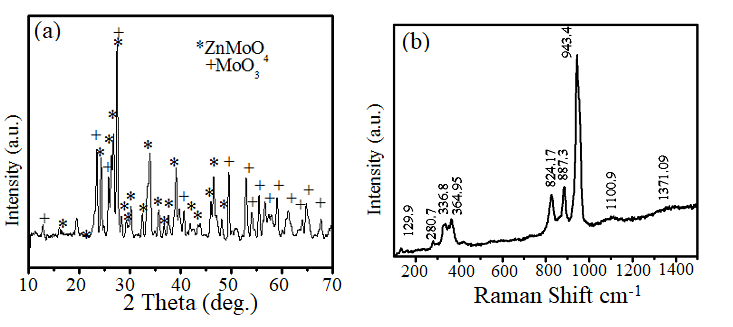
Figure 2: (a) X-rays Diffraction spectrum of AP-ZMO (b) Raman spectrum of AP-ZMO.
GC-MS profile of AP-ZMO is presented in figure 1b, which identified cyclobutanol (C4H8O) presence in AP-ZMO at particular retention times (RT) of 5.18 and 5.7. The presence of organic peaks in figure 1b is associated with phytocompounds of PEAP as described in our previous studies.23-24 Thus, FTIR and GCMS are proposing the presence of A.pindrow phytocompounds in the synthesized AP-ZMO.
Figure 2a depicts the p-XRD analysis of bio-organic compounds present in AP-ZMO. The diffraction patterns presented in figure 2a demonstrates well-defined most prominent peaks of MoO3 at 2theta (?)=25.8(040), 27.48(021), 52.89(211). The diffraction pattern indicates the presence of orthorhombic MoO3 (ICDD:00-005-0508), and ZnMoO4 (Zinc-Molybdenum Oxide) (ICDD:00-035-0765). Giving main peaks at 24.362° (120), 24.921° (111), 27.488° (021), 33.9537° (-212), 39.1236° (-3-21), and 49.4792° (-3-13). The Debye Scherrer's equation-based calculation40 of crystallite size revealed the nano crystallite size of AP-ZMO in the range of 30-32 nm. However, the presence of AP-ZMO phase validated by p-XRD, was also elucidated by Raman analysis (figure 2b). Figure 2b shows the chemical analysis of AP-ZMO carried out by Raman spectroscopy displaying Raman vibration modes of AP-ZMO. The vibration peaks above 1000 cm-1 in figure 2b suggest the presence of PEAP phyto-compounds in consistent with the reported literature.23-24 Certain other vibrations can also be noticed at 300 to 3500 cm -1, signifying Zn and Mo.24 However, an apparent change in the spectrum of AP-ZMO can be seen within the 300-1500 cm-1 range because of the addition of PEAP phytoconstituents. The results of the Raman analysis are in good agreement with the findings of FT-IR and GCMS analysis.
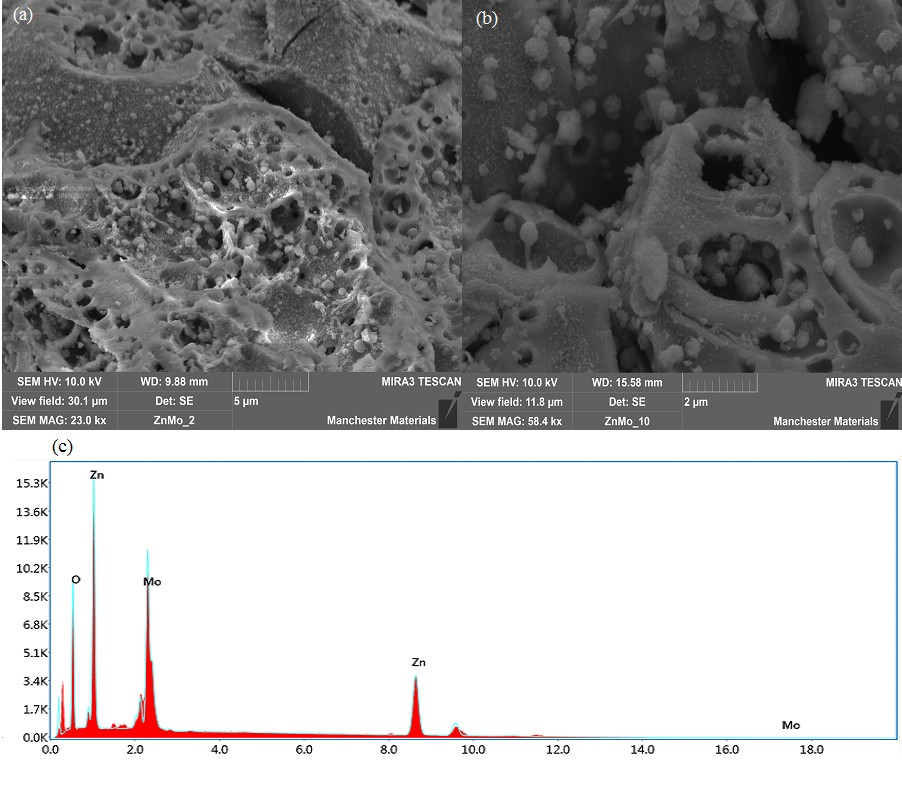
Figure 3: (a-b) FESEM of PEAP-assisted AP-ZMO and (c)Elemental analysis of AP-ZMO using EDX.
FESEM images of obtained AP-ZMO are shown in figure 3, and a hierarchical structure is observed at different magnifications along with the presence of spherical particles. The AP-ZMO structure may have formed due to the annealing of the sample containing organic moieties in the air. The removal of unreacted organic compounds/residue during the calcination process may have resulted in the formation of a porous hierarchical structure.
The catalytic degradation role of PEAP-prepared binary composite was investigated to degrade MO in aq. solution in the presence of sunlight (SLMO) as well as under dark ambient (DAMO) conditions. A blank solution was also run to test the catalytic behavior of AP-ZMO. Starting concentration of MO was 1 mg /mL and the catalyst loading was 2mg/10 mL while the absorbance was noted at different time intervals starting from 0 minutes - 20 minutes (figure 4). The calibration curves for MO were detected by noting the λmax for different intervals of time (figure 4). Absorption maxima were found at 464 nm23-24 and it was used in calculating the percentage degradation (figure 4) of MO dye (equation 1).
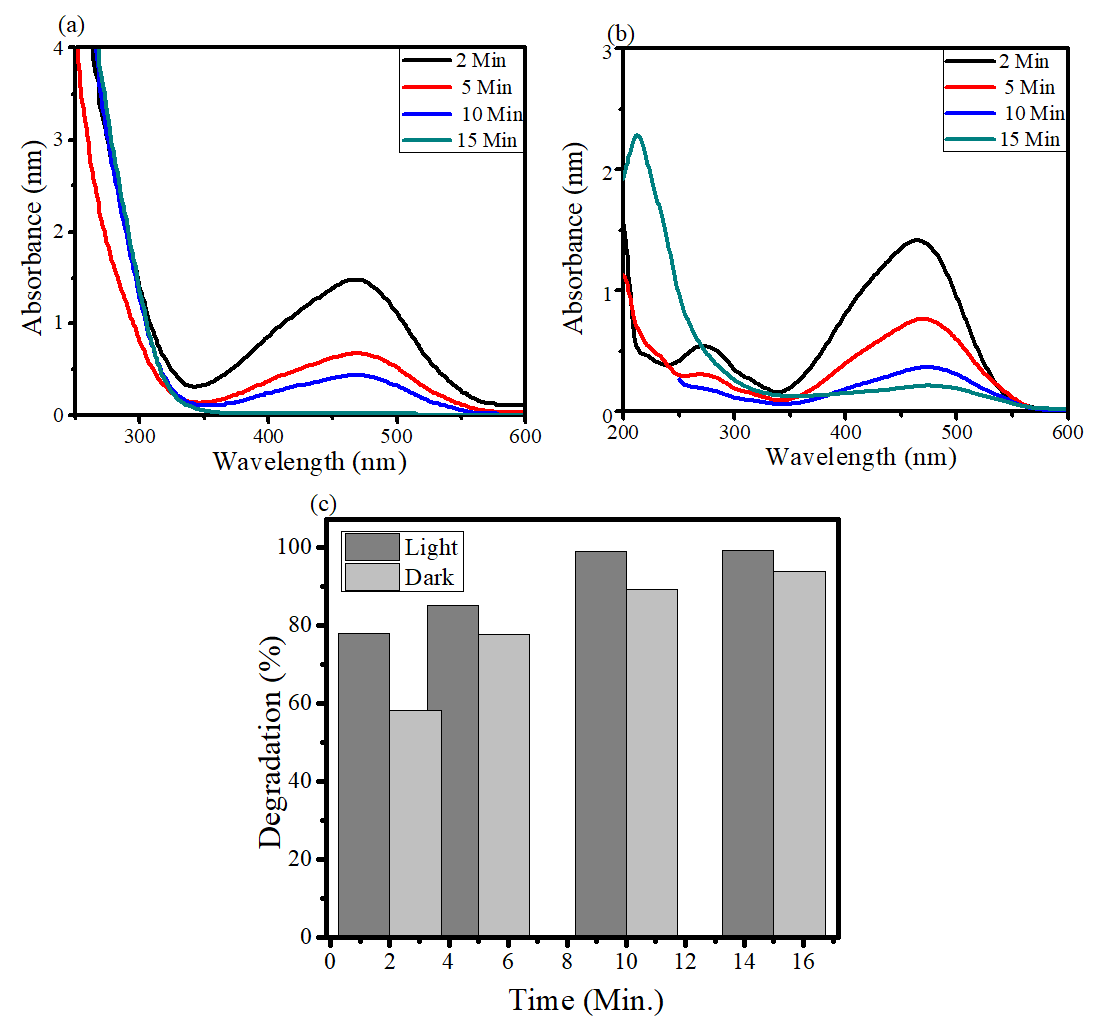
Figure 4: UV-Vis absorbance of dye degradation by AP-ZMO (a) SLMO (b) DAMO, and (c) Degradation (%) of SLMO and DAMO by AP-ZMO composite.
In figure 4a-b, the broader sharper peak at 464 nm along with a delicate peak at 270 nm can be noticed. The absorption band at 464 nm is because of n-π*transition1 of the azo bond. However, as the time interval increases, the absorbance band significantly decreases because of the azo bond breakage by the catalyst under SLMO as well as DAMO. The weaker peak observed at 270 nm is because of aromatic intermediate intrusions given by the azo bond. UV-Vis spectral interpretations (figure 4) have shown prominently that a significant reduction at 464 nm absorption peak can be seen in the case of SLMO light reaction while a comparatively less DAMO condition. Figure 4a also showed breakage of azo bonds within 10 minutes under light, and a continuous absorption peak is seen below 300 nm, whereas the diminishing peaks for azo bonds below 300 nm were also clearly seen after 10 minutes under dark (figure 5b) demonstrating the efficient behavior of AP-ZMO as photocatalyst.
Table 1: Degradation percentage of MO under SLMO and DAMO reactions in comparison with blank.
|
Time (min) |
Degradation (%) |
Blank (%) |
||
|
SL-MO |
DA-MO |
Under Light |
Under Dark |
|
|
2 |
78.08 |
58.25 |
0 |
0 |
|
5 |
85.2 |
77.6 |
2 |
0 |
|
10 |
98.88 |
90 |
5 |
2 |
|
15 |
99.2 |
93.7 |
5 |
0.5 |
With the help of absorbance maxima at 464 nm at different intervals of time, degradation % was calculated as presented in figure 5c and table 1. Figure 4c shows AP-ZMO exhibited an efficiency of 98.88% under light for MO degradation till 10 minutes and 90% degradation was found under dark conditions for the same time interval. However, till next time interval of 15 minutes, degradation was raised to 99% and 94% for both SLMO and DAMO respectively. The porosity and hierarchical morphology of AP-ZMO are the factors behind this extraordinary catalytic performance (figure 3). Table 1 also shows that at 5 minutes, 85% degradation for SLMO and 77 % for DAMO was observed. These findings validated the AP-ZMO as an excellent catalyst under dark conditions without solar energy. Therefore, organic compounds based AP-ZMO is an efficient catalyst for MO degradation, under both light and dark reaction conditions.
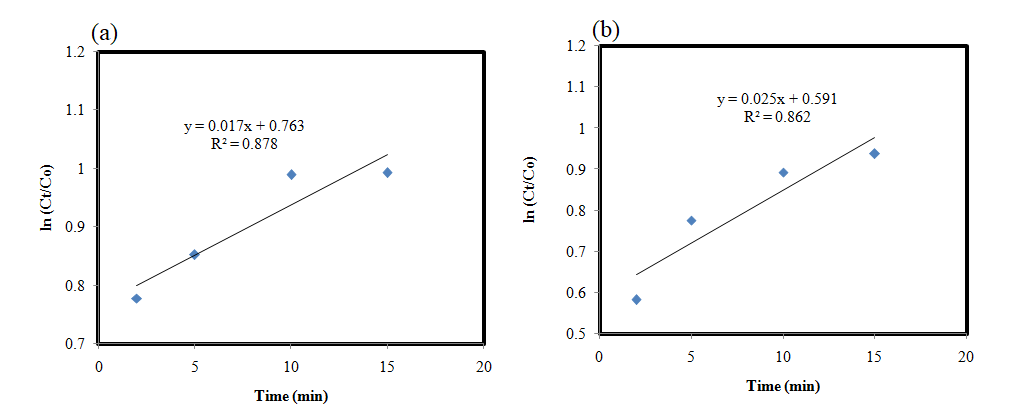
Figure 5: ln (Ct/Co) vs time-catalyticreactions shown by AP-ZMO (a) under SLMO (b) in DAMO.
Reaction kinetics (ln (Ct/Co) vs time) for describing the order of catalytic reactions has been given in figure 5. Declination of MO was noted to be Pseudo first-order kinetics in all experiments. ln (Ct/Co) vs time gave a direct linear relation with R2 < 1. The regression value for AP-ZMO was determined as 0.87 in the case of light and in dark it was about 0.86. The values of R2 for SLMO and DAMO are signifying the good fitted Pseudo first-order kinetics as increasing reaction time and decreasing degradation percentages are linear fitting suggesting the stable efficiency of the catalyst under dark and light conditions. Thus, regression analysis illustrated AP-ZMO as a significant stable catalyst under SLMO and DAMO reactions.
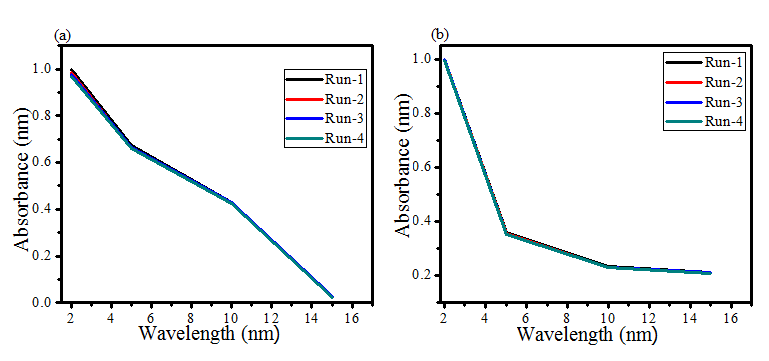
Figure 6: Reusability ofAP-ZMO composite catalyst (a) SLMO (b) DAMO.
The catalytic activity of AP-ZMO was sustained for four turns of experiments by recollecting the catalyst via centrifugation (6000 rpm, 10 minutes), washing and drying at a temperature of 80 oC. AP-ZMO composite showed good stability in all four turns as shown in figure 6 significant degradation efficiency. The excellent catalytic efficiency of 97.5, as well as 94.1 %, was attainable even after the fourth time repetition of the experiment under both SLMO and DAMO respectively. The AP-ZMO revealed enhanced stability for its reusability as compared to the literature, for example, the degradation efficiency of Cu2(OH)3NO3/ZnO,3 1 and ZnO/Ag2O,32 were reported to be maintained till the three cycles of experiments. Ta and coworkers reused AgNWs/ZnO NRs/AgNPs for four repetitions (run) having 98% degradation of MO in 40 minutes.2 However, in the current study, enhanced AP-ZMO efficiency was retained within 15 minutes after four cycles. The obtained stability was credited to the incorporated oxygen and carbon compounds given by phyto PEAP template. In the current study, after the fourth cycle, the catalyst showed better performance in SLMO than DAMO (figure 6a,b) indicative of intact as well as photostable AP-ZMO composite. Such catalyst’s stability is tremendously vital for the practical application of a catalyst. This photostability is because of photo-generated electrons as well as holes of mixed MOs along with bioactive compounds of PEAP.
Figures 4 and 5 reveal that AP-ZMO catalyst showed an effectual catalytic role just in 15 minutes under both SLMO as well as DAMO. No effect of light was witnessed on catalyst performance in the first 10 minutes. Srikhaow31 reported the same activity of the catalyst by catalytic wet oxidation (CWO) process where organic compounds go through aerial oxidation above the catalyst surface. CWO phenomenon is reliant on more oxygen vacancies to degrade MO. In this report, due to combined (binary) metal oxides, oxygen vacancies were improved to aid CWO. Furthermore, oxygen-containing PEAP compounds as confirmed by GC-MS and FTIR have augmented the catalytic activity. Thus, in the current work, CWO phenomenon is accountable for the degradation of MO under DAMO, while the efficiency of CWO by MO was completely reliable on oxygen concentration in prepared catalysts. The heterojunction metal oxides and inserted phyto-organic groups competently improved catalytic potential of AP-ZMO catalyst even in absence of light or any chemical stimulants with illustrated higher reusability.
Herein, we have established a sustainable synergistic approach of inorganic-organic patterned spherical-shaped AP-ZMO structures prepared via organic functionalities of A.pindrow. We have introduced a facile greener route for AP-ZMO and fruitfully functionalized AP-ZMO by organic groups which were demonstrated to be active mediators responsible for enhancing active sites of AP-ZMO catalyst for effectual degradation of MO in aqueous media along with catalytic effectiveness of 99% in light and 94% in the dark environment within 15 minutes. Moreover, a reasonable degradation activity of 85 % and 74 % were also seen in a short time interval i.e., 5 minutes under light and dark environments, respectively. This admirable role of the catalyst was credited to synergetic effects given by porosity and more active sites provided by C-O containing functional groups. Therefore, we have presented an efficient implication of phyto organic compounds consequential AP-ZMO catalyst for water remediation proposing a viable approach to prepare unique sustainable catalysts for the degradation of organic pollutants under ambient conditions without stimulants.
The authors declare that they have no known conflict of interest that could influence the work reported in this paper.
The authors acknowledge the Higher Education Commission of Pakistan, Department of Environmental Sciences, Fatima Jinnah Women University Rawalpindi Pakistan, and the School of Materials, The University of Manchester U.K.
Keywords: Phytotemplate,Metal Oxides,Methyl Orange,Catalysis,Degradation,Dark conditions
Materials Innovations (MI) is an interdisciplinary journal devoted to significant experimental and theoretical findings on the synthesis, structure, charachterization, processing and applications of materials. Materials Innovations is dedicated to publishing peer reviewed novel, cutting edge reports of broad interest to the materials science community.