
Full Html
Vol 2 Issue 3 (Special Issue)
Tin Oxide Supported on MnO2 as Efficient Catalyst for Reduction: Kinetic Analysis and Their Application as Heterogeneous Catalyst
Pages: 83-91
Doi: 10.54738/MI.2022.2303
Doi URL: http://doi.org/10.54738/MI.2022.2303
Ata Ullah 1 , Lutfur Rahman 1 , Syed Zajif Hussain 2 , Muhammad Bilal Yazdani 1 , Asim Jilani 3 , Dayum Iqbal Khan 1 , Musadiq Zeshan Nasir 1 , Waheed S Khan 1 , Irshad Hussain 2 , Asma Rehman?? 1
1 National Institute for Biotechnology and Genetic Engineering (NIBGE), Faisalabad (38000), Pakistan
2 Constituent College Pakistan Institute of Engineering and Applied Sciences, Faisalabad, Pakistan
3 Department of Chemistry and Chemical Engineering, SBA School of Science & Engineering (SBASSE), Lahore University of Management Sciences (LUMS), DHA, Pakistan
4 Center of Nanotechnology, King Abdul Aziz University, Jeddah, 21589, Saudi Arabia
Recently, metal oxides-based nanomaterials have been widely used for catalytic reduction of nitro-aromatic compounds, which are notorious for their carcinogenic nature. The current study reports Sn-doped MnO2 as an efficient catalyst for the reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP). The FE-SEM characterization of SnO2-doped MnO2 revealed the diffused flower-like morphology. Further, the XPS survey scans were performed to investigate the binding energies, oxidation states, and elemental compositions of both MnO2 and Sn-doped MnO2. Kinetics analysis revealed that the catalytic reduction (> 98.8%) of 4-NP into 4-AP by Sn-doped MnO2 in the presence of NaBH4 occurs within four min, following pseudo-first order kinetics. Importantly, no observable deactivation of catalytic efficiency was noticed even after five cycles. Our strategy of loading SnO2 on the surface of semiconductor nanostructures offers a versatile approach to enhance the catalytic performance, stability, and recyclability which may further promote their practical industrial application.
Keywords
Industrial effluent, Carcinogenic, Catalytic reduction, Sn doped MnO2, 4-Nitrophenol
Advancement in metal oxides-based nanomaterials have garnered their application in several fields, including catalysis.1 Metal oxides are used for the development of various catalysts due to their unique physicochemical properties such as high stability, multiple active-sites, and large surface-area. Such nano-catalysts have sufficient bandgap to catalyze several chemicalreactions.2 Among these metal oxides, manganese dioxide (MnO2) is considered as a promising material for designing the catalysts for efficient reduction of aro?matic compounds, such as 4-Nitrophenol (4-NP). It is because of their relative abundance, environmental compatibility, high surface-reactivity and chemical? stability.3 Morphology of such materials can be tuned into various shapes, such as nanosphere4, nanowires5, and nanotubes6, etc.
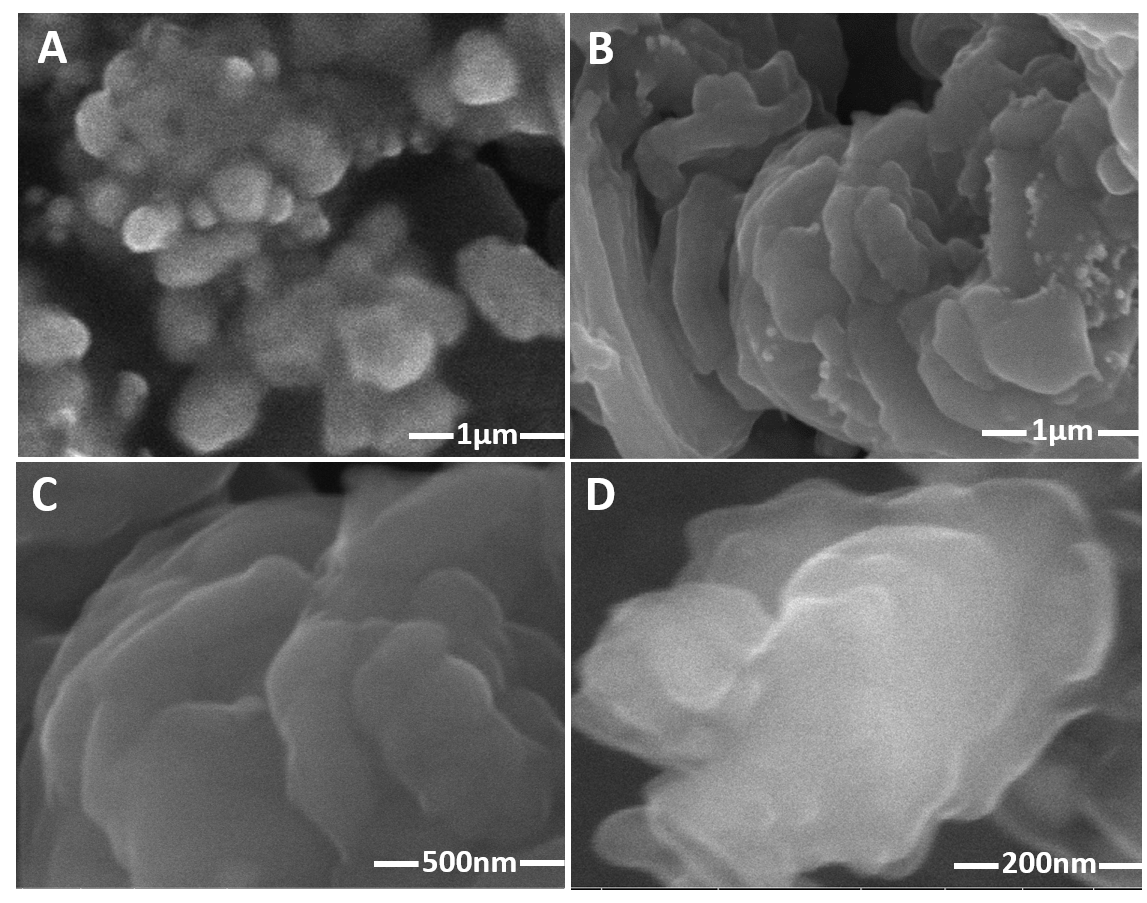
Figure 1: Field emission scanning electron micrograph of the designed catalyst (a) MnO2 NPs. (b, c, d) Sn-doped MnO2 morphologies at different resolutions.
However, their catalytic activity has been restricted due to the fast recombination of electron-hole pairs (ehp).7 Further, metal oxide nanoparticles (NPs) can agglomerate with each other and remain unstable due to their high-surface energy. In addition, from the reaction mixture, these NPs cannot be easily separated and thus difficult to recycle. Therefore, numerous efforts have been made to stabilize metal oxide NPs to prevent their agglomeration and enhance their stability/recyclability.8 Surface-capping of metal oxides NPs effectively prevent their agglomeration and maintain their stability. Similarly, these NPs may be loaded on supporting materials to design heterogeneous catalysts that exhibit high catalytic activity and are easier to recycle and reuse.9 Another approach is doping, which is the most promising, and give multiple properties of two or more NPs in a single composite resulting in extraordinary catalytic activity, stability, and recyclability.10
The current study reports a convenient and facile approach for the synthesis of Sn-doped MnO2 based heterogeneous catalysts with unique morphology and proficient catalytic activity. Tin-oxide (SnO2) is an n-type metal oxide semiconductor that exhibits high carrier density, oxygen vacancy, and chemical stability. Due to these properties, it can potentially catalyze several reactions in an environmental interest.11 Therefore, Sn-doped MnO2-based composite may have an excellent interface to prevent ehp recombination. The possible reason behind it could be the creation of surface defects and oxygen vacancies on the surface of SnO2. Thus, increasing in the catalytic performance, stability and recyclability of the designed hybrid catalyst.11,12
On the other hand, rapid industrialization has led to the contamination of water which is not only vital for human survival but also necessary for sustainable agriculture.13 Effluents from several, chemical, pharmaceutical, pulp/paper and tannery industries are frequently being discharged into aquatic bodies without prior treatment, which causes severe environmental concerns.14 These effluents contain heavy metals, recalcitrant dyes and other aromatic compounds.15 Among them, 4-NP is an essential precursor in the synthesis of synthetic dyes, paint, pesticides, herbicides explosive, drugs, wood preservatives, etc.16 Therefore, effluents discharged from these chemical industries are contaminated with 4-NP, which is highly carcinogenic for animals and induces toxicity in living tissues via the redox process.17 Therefore, the detoxification of 4-NP in aqueous bodies via reduction into their lesser toxic counterpart has significantly attracted the global interests.17
To address the above-mentioned environmental challenges, we report a facile hydrothermal approach for the synthesis of Sn-doped MnO2 hybrid composite, as a promising catalyst for 4-NP reduction. The as-synthesized catalyst has been characterized through Field-Emission Scanning Electron Microscope (FE-SEM) and X-ray Photoelectron Spectroscopy (XPS). Their catalytic efficiency has been measured spectrophotometrically at different concentrations of 4-NP and sodium borohydride (NaBH4). Additionally, the recyclability of Sn-doped MnO2 catalyst has also been checked for 5-cycles that demonstrate its excellent potential for recyclability and reusability.
All analytically grade chemicals i.e., potassium permanganate (KMnO4) 98+ % from ACROS, anhydrous Tin (IV) chloride (SnCl2.4H2O) 98 % from Alfa Aesar, ethanol (absolute), hydrochloric acid (37 %) from Sigma-Aldrich, hydrogen peroxide (H2O2), and poly (vinyl)alcohol (PVA) were purchased from ACROS. All the solutions were prepared in deionized water (ρ = 18 MΩ cm from the Millipore Milli-Q system.
Manganese dioxide (MnO2) nanostructures were synthesized by modifying the previously reported method.18 In a typical experiment, 0.048 g of KMnO4 was added into 50 mL of HNO3 (1.3M). After 30 min magnetic stirring, the solution was transferred to a Teflon-lined autoclave and hydrothermally treated at 80 °C for 5 hours. The product was recovered through centrifugation (3000 rpm), and washed several times with ethanol, and deionized water sequentially. Then, the prepared catalysts were vacuum dried at 60 ?C overnight and stored in a desiccator for further use.
For the synthesis of Sn-doped MnO2, as-synthesized MnO2 nanostructures were allowed to a second low-hydrothermal treatment in the presence of SnCl2.4H2O ranging from 1:1 to 1:9 Sn/Mn atomic weight ratios.19 Briefly, a specific amount of thoroughly sonicated MnO2 nanostructures were added to 20 mL acidic solution (0.7 mL of 38%) of SnCl2.4H2O (0.15 g). The mixture was magnetically stirred for 30 min. Then, transferred it to 100 mL Teflon-lined stainless-steel autoclave followed by hydrothermal treatment at 90 ?C for 5 hours. The as-synthesized Sn-doped MnO2 were recovered through centrifugation, vacuum dried at 70 ?C and stored in a desiccator for further studies.
The morphology of the synthesize nanomaterials were explored through Field Emission-Scanning Electron Microscopy (FE-SEM). Further, the compositional information and binding energy of Sn-doped MnO2 were determined through X-ray Photoelectron spectroscopy (XPS).
The catalytic activities of both MnO2 nanostructure and Sn-doped MnO2 were investigated using 4-NP as a model pollutant. In a typical reaction, 2.7 mL of 4-NP (0.1 mM) was mixed with 0.3 mL of freshly prepared NaBH4 (0.1 M), followed by the addition of catalyst in the range of 1-5 mg mL-1 under optimized reaction conditions at room temperature. The catalytic reduction of the 4-NP was spectrophotometrically monitored in the range of 200-700 nm with respect to time (t). The recyclability of as-synthesized catalyst was also checked as follows: once the reaction was completed, the catalyst was recovered through centrifugation, washed with 10 % ethanol solution, and vacuum dried at 70 ?C for 5-6 h. The dried catalysts were then again used for the subsequent reactions to check their reusability and stability.
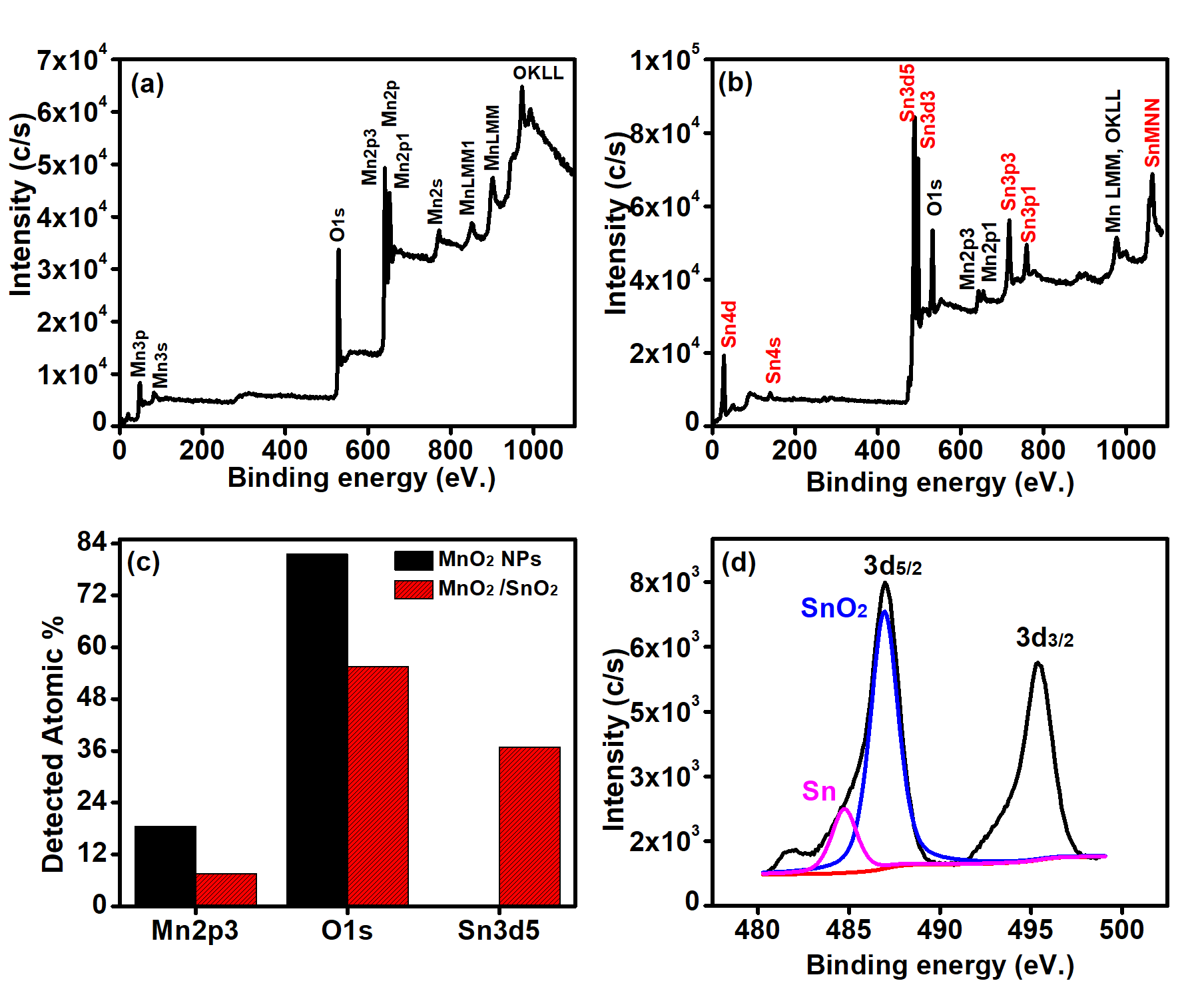
Figure 2: (a-c) Survey scan and compositional analysis of MnO2 and Sn-doped MnO2, while (d) Sn3d5 analysis of Sn-doped MnO2.
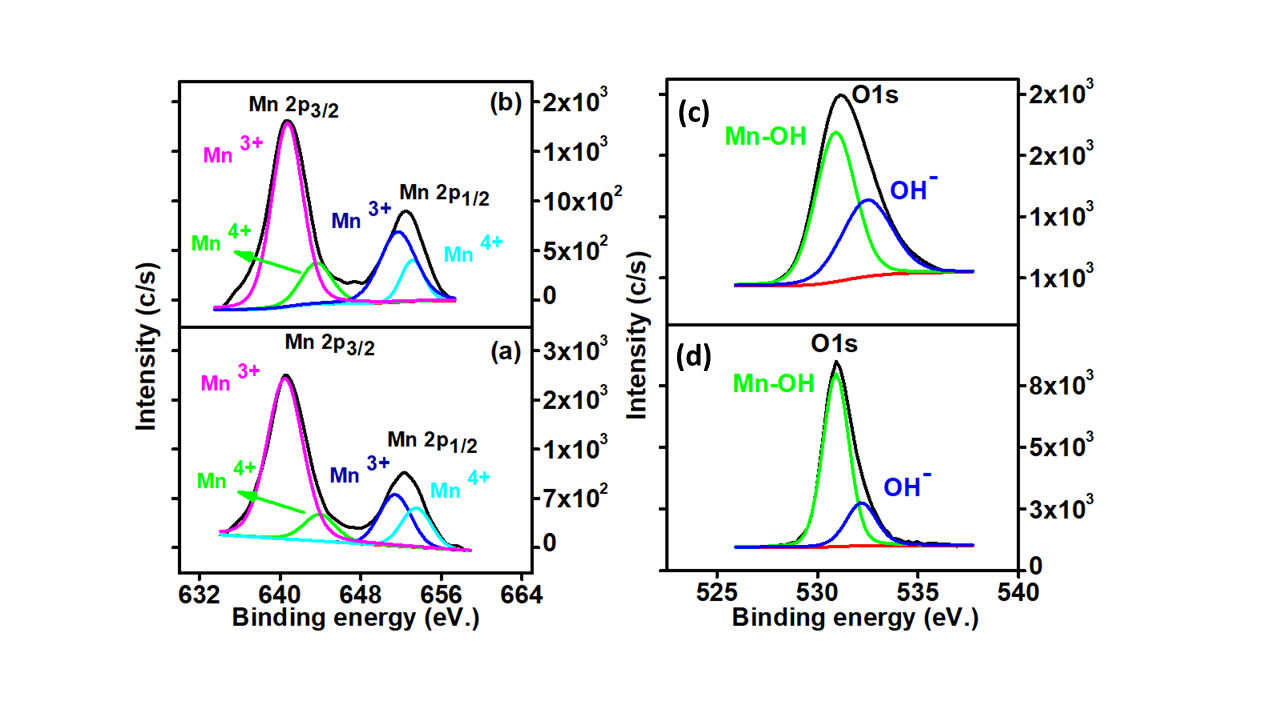
Figure 3: Mn2p analysis for (a) MnO2 nanostructures (b) Sn-doped MnO2 (c-d) O1s functional groups analysis for (c) MnO2 nanostructures and (d) Sn-doped MnO2.
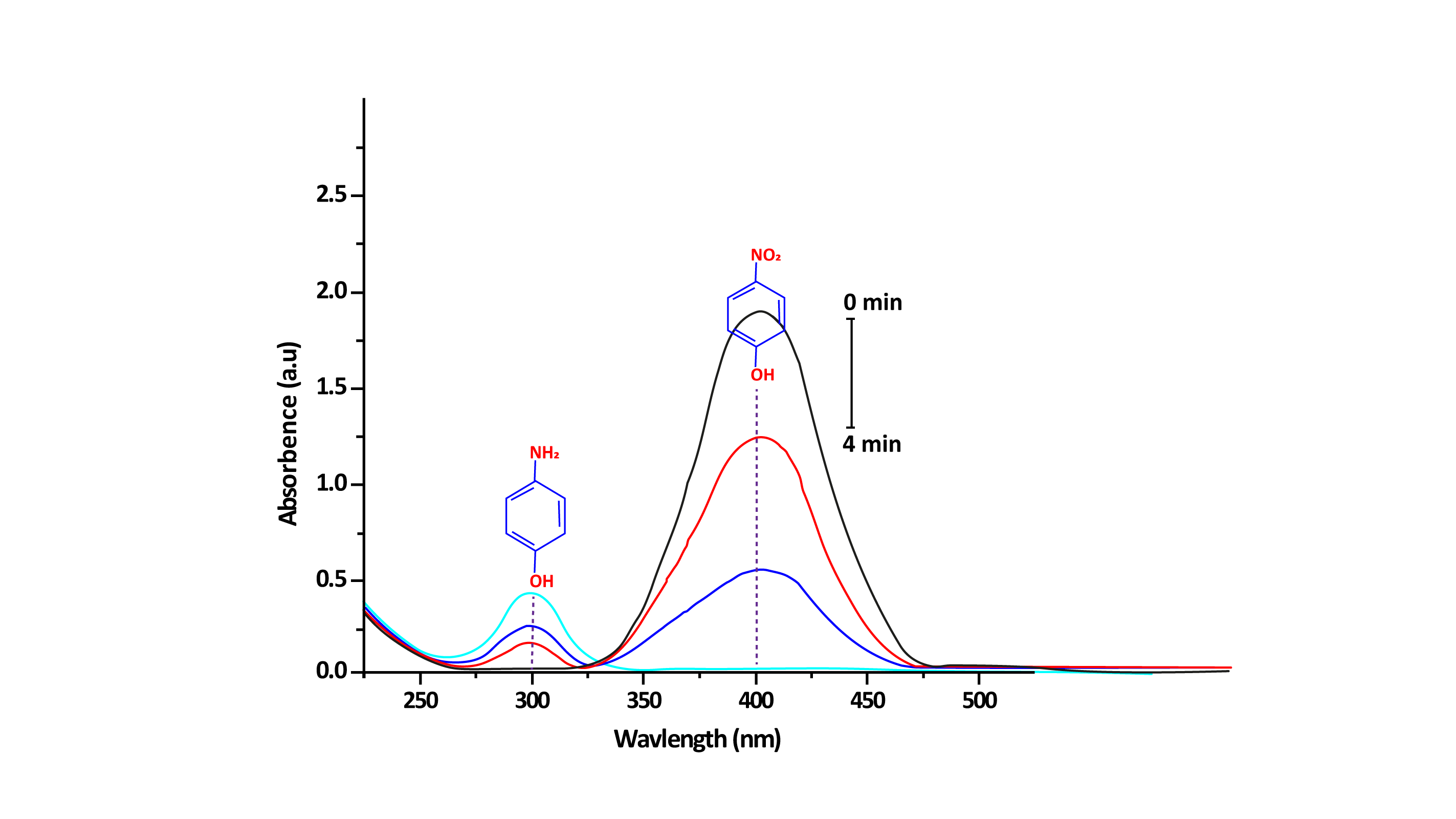
Figure 4: UV-Vis absorption spectra of the catalytic reduction of 4-NP in the presence of Sn-doped MnO2, using NaBH4 as electrons donor.
The reduction kinetics of 4-NP were calculated from the UV-Vis absorption spectra of the reaction solution, which reveal that the reduction kinetics follow pseudo-first-order. Pseudo-first-order kinetics can be described as ln (Ct/Co) where Co and Ct are the concentrations of 4-AP at time zero and t, respectively. The ln (Ct/Co) was plotted against reaction time (t), which exhibited the best linear correlation (R2 > 0.996) as shown in Figure 5(a). The rate constant (Kapp) was calculated from the slope of ln(Ct/Co)-t , which revealed that the Sn-doped MnO2 had efficient catalytic reduction activity with Kapp > 4.7884 min-1 as compared to MnO2 alone (Kapp = 0).

Figure 5: (a) Plot of ln (Ct/Co) against the reaction time ‘t’ for MnO2, Sn-doped MnO2 under 1M NaBH4, 0.1mM 4-NP, respectively at room temperature, (b) Plot of Kapp against amount of Sn-doped MnO2 under optimized conditions, (c, d) are the effect of 4-NP and NaBH4 concentrations on the rate constant Kapp.

Figure 6: (a) Plot of 4-NP % reduction against reaction time; (b) recyclability and reusability properties of the Sn-doped MnO2 catalyst under optimized reaction condition.
The FE-SEM morphology of MnO2 showed their diffused flower-like morphology as shown in Figure 1(a). Such flower-like morphology for MnO2 has also been reported by Rivera-Lugo and co-workers.18 Our study shows that at low hydrothermal temperature and lesser treatment time (5-6 h), MnO2 exhibits flower-like morphology. However, at high temperature and longer treatment time, the flower-like morphology transforms into rod-like structures. The FE-SEM micrograph of Sn-doped MnO2 shows flower-like morphology, which is comprised of large, thick petal-like layers (Figure 1 (b-d)). This may indicate that second-low hydrothermal treatment of MnO2 in the presence of Tin (IV) chloride (acidic condition) has improved their diffused flower-like morphology with well-defined, large and thick layers, like petals. Such transformation in morphology has also been demonstrated by Lan and co-workers.20 Zhao et al have further explained that the flower-like morphology provides large surface area and enhance catalyst’s activities. Further reported that catalysts with flower-like morphology possesses more atoms at the edges and corners, thus provides more active sites for the interactions with reactants.21
The surface compositions of as-synthesized MnO2 nanostructures and Sn-doped MnO2 were investigated using XPS. The survey scan of MnO2 nanostructure revealed the presence of Mn2p3 (18.5 %) and O1s (81.5%) without any residual impurities (Figure 2(a)). Further, the survey scan of Sn-doped MnO2 (Figure 2(b)) confirms the presence of Sn3d5 (36.9%) along with Mn2p3 (7.6%) and O1s (55.5%). The graphical representation of each detected element of MnO2 nanostructure and Sn-doped MnO2 is shown in Figure 2(c).
Moreover, the chemical state analysis of Sn3d5, Mn2p3 and O1s were performed to determine the functional groups. The Sn3d5 analysis of Sn-doped MnO2 (Figure 2(d)), Sn3d5 has two peaks around 486.97 eV and 495.47 eV that are attributed to the 3d5/2 and 3d/3/2, respectively. Further, the difference between these two peaks were noticed around 8.5 eV. Moreover, the peak fitting of 3d5/2 disclosed the manifestation of two peaks around 484.74 eV and 486.94 eV, which is recognized as SnO2 and Sn in metallic form.22 Moreover, the contribution of SnO2 was around 81.34 %, while 18.66% for Sn metallic.
Mn2p analysis for MnO2 nanostructures show (Figure 3(a)) the appearance of two main peaks around 640.50 and 651.41 eV which are attributed to the 2p3/2 and 2p1/2, respectively. Further, the peak fitting of 2p3/2 and 2p1/2 revealed four peaks around 640.49 eV, 643.95, 651.41 and 653.50 eV respectively for Mn3+, Mn4+ (2p3/2 ) and Mn3+, Mn4+ (2p1/2 ).22 Moreover, a change in Mn3+ and Mn4+ was observed after the addition of SnO2 (Figure 3(b)).
O1s spectra of MnO2 nanostructures (Figure 3(c)) show the presence of two peaks around 530.90 eV and 532.3 eV, which are attributed to Mn-OH and OH-, respectively. The contribution of Mn-OH changed from 76.80 % to 60.70 % with the addition of Sn to MnO2 (Figure 3(d)).23-24 However, an increase in the OH- was noticed from 23.20 % to 39.30 % is favorable for the enhanced catalytic activity of these materials.25
The catalytic activity of the Sn-doped MnO2 was explored for the reduction of 4-NP into 4-aminophenol (4-AP), using sodium borohydride (NaBH4) as a source of electron donor. Catalytic reduction of 4-NP was monitored by UV-Vis absorption spectra of the solution (Figure 4). A maximum absorption spectrum of 4-NP ions in solution was recorded at 400 nm, when NaBH4 was added to the 4-NP solution. Once, the Sn-doped MnO2 catalyst was added to 4-NP solution, a significant decrease was observed in the intensity of absorption peak at 400 nm, while an increase in absorption peak at 300 nm was recorded that indicates the conversion of 4-NP to 4-AP. Moreover, the catalytic reduction was also observed visually by observing the fading of the greenish color of 4-NP solution to transparent.26 As-synthesized Sn-doped MnO2 catalysts have shown excellent reduction activity and completed the reduction within 4 minutes, however MnO2 nanostructures haven’t shown significant catalytic activity. Thus, the best catalytic activity of Sn-doped MnO2 is due to the synergistic effect of both metal oxides, namely SnO2 and MnO2. The reported literature has mentioned that Sn-doping on MnO2 heterostructure may decreases their bandgap. Due to which the electrons are easily ejected for MnO2 to produced electron-hole pairs that impart proficient catalytic activity to Sn-doped MnO2 catalyst.27
Further, the 4-NP reduction kinetics were explored at different reaction conditions. The amount of Sn-doped MnO2 was changed in the range of 1-5 mg, while keeping NaBH4 (1 M) and 4-NP (0.1 mM) concentrations constant. Under these optimized conditions, the Kapp increased linearly with increasing the amount of catalyst (Figure 5(b)). It is obvious, that increase in catalyst amount provide more active sites for the reduction process. In the case of increasing NaBH4 concentration from 0.025 to 0.1 M, an increase in Kap p occurred; though no change in Kapp was observed when the concentration increased from 0.15 M to 0.2 M (Figure 5(c)). Similarly, the effect of 4-NP concentration (0.05-0.2 mM) was investigated, and the results are shown in Figure 5(d). The Kapp increases as the 4-NP concentration increases from 0.05 to 0.15 mM, but beyond 0.15 mM concentration Kapp decreases. However, these nonlinear relationships between Kapp with NaBH4, along with a fall in Kapp by increasing the 4-NP concentration shows that both reactants compete with each other on the surface of Sn-doped MnO2 catalyst. Similar relation between rate constant and nitrophenol concentration was documented by Mona and Reza Gholami, using CeO2 nanorods supported on CuNi nanoparticles.28
The percent (%) reduction of 4-NP to 4-AP by the as-synthesized catalysts in the presence of NaBH4 were calculated using the equation; η=1-Ct/Co×100 (Figure 6(a)),29 which showed > 98% reduction of 4-NP into 4-AP within 4 min. The recyclability and reusability of Sn-doped MnO2 were checked for five consecutive cycles under optimized conditions (4-NP (0.1 mM), 0.1 M NaBH4 at RT) (Figure 4(b)). The results showed that in each consecutive cycle the reduction time increases slightly i.e., up to 15-30 sec, that might be due to the minute loss of catalyst during recovery processing (centrifugation, washing and drying).30
A facile hydrothermal approach was used for the synthesis of hybrid Sn-doped MnO2 catalyst. The flower-like morphology of Sn-doped MnO2 with diffused petals showed that the deposition of specific amount of Sn on MnO2 nanostructure has improved the petal-like layers of MnO2. XPS study confirmed that the Mn-OH reduced from 76.80 % to 60.70 % due to the addition of Sn on MnO2, which depict the successful deposition of Sn on MnO2 nanostructure. The pure MnO2 nanostructures were found catalytically inactive but the designed Sn-doped MnO2 have reduced 4-NP into 4-AP (>98.8%) only in four minutes with high catalytic activity even after five cycles, thus demonstrating their effective reusability.
The authors declare not conflict of interest.This study was funded by the Higher Education Commission (HEC) of Pakistan under the head of NRPU Project # 6118). The Principal investigator (PI) of this project was; Dr. Asma Rahman, Senior scientist at National Institute of Biotechnology and Genetic-engineering (NIBGE), Faisalabad. The authors highly acknowledge the financial support of the HEC, Pakistan.1
Barua, Ranjit, Datta, Sudipto & Das, Jonali . 2020. Application of Nanotechnology in Global Issues. Advances in Human Services and Public Health 292–300.
Narayan, Neel, Meiyazhagan, Ashokkumar & Vajtai, Robert . 2019. Metal Nanoparticles as Green Catalysts. Materials 12(21):3602.
Chiam, Sin-Ling, Pung, Swee-Yong & Yeoh, Fei-Yee . 2020. Recent developments in MnO2-based photocatalysts for organic dye removal: a review. Environmental Science and Pollution Research 27(6):5759–5778.
Srither, S.R., Karthik, A., Murugesan, D., Arunmetha, S., Selvam, M. & Rajendran, V. . 2015. Electrochemical capacitor study of spherical MnO2 nanoparticles utilizing neutral electrolytes. Frontiers in Nanoscience and Nanotechnology 1(1):13–20.
Tehseen, Bushra, Rehman, Asma, Rahmat, Muniba, Bhatti, Haq Nawaz, Wu, Aiguo, Butt, Faheem K., Naz, Gul, Khan, Waheed S. & Bajwa, Sadia Z. . 2018. Solution growth of 3D MnO2 mesh comprising 1D nanofibres as a novel sensor for selective and sensitive detection of biomolecules. Biosensors and Bioelectronics 117:852–859.
Chalkidis, A, Jampaiah, D, Amin, M H, Hartley, P G, Sabri, Y M & Bhargava, S K . CeO2-Decorated?-MnO2 Nanotubes: A Highly Efficient and Regenerable Sorbent for Elemental Mercury Removal from Natural Gas. Langmuir 2019(25):8246–8256.
Khan, M.A., Nadeem, M.A. & Idriss, H. . 2016. Ferroelectric polarization effect on surface chemistry and photo-catalytic activity: A review. Surface Science Reports 71(1):1–31.
Cartwright, Anthony, Jackson, Kyle, Morgan, Christina, Anderson, Anne & Britt, David W. . 2020. A Review of Metal and Metal-Oxide Nanoparticle Coating Technologies to Inhibit Agglomeration and Increase Bioactivity for Agricultural Applications. Agronomy 10(7):1018.
Ma, Hui-Chao, Kan, Jing-Lan, Chen, Gong-Jun, Chen, Cheng-Xia & Dong, Yu-Bin . 2017. Pd NPs-Loaded Homochiral Covalent Organic Framework for Heterogeneous Asymmetric Catalysis. Chemistry of Materials 29(15):6518–6524.
Yang, Yuwen, Luo, Sha, Guo, Shuailong, Chao, Yunxiu, Yang, Hongwei & Li, Yuxiu . 2017. Synthesis of Au nanoparticles supported on mesoporous N-doped carbon and its high catalytic activity towards hydrogenation of 4-nitrophenol to 4-aminophenol. International Journal of Hydrogen Energy 42(49):29236–29243.
Periyasamy, Muthaimanoj & Kar, Arik . 2020. Modulating the properties of SnO2 nanocrystals: morphological effects on structural, photoluminescence, photocatalytic, electrochemical and gas sensing properties. Journal of Materials Chemistry C 8(14):4604–4635.
Shao, Meng, Liu, Jiajia, Ding, Wenjie, Wang, Jingyu, Dong, Fan & Zhang, Jiatao . 2020. Oxygen vacancy engineering of self-doped SnO2 nanocrystals for ultrasensitive NO2 detection. Journal of Materials Chemistry C 8(2):487–494.
Kilic, Z . The importance of water and conscious use of water. Int J Hydro 2020(5):239–241.
SI, Borrely, JM, Rosa, NF, Boiani, VSG, Garcia & AL, Sousa . 2018. Emerging pollutants, related toxicity, and water quality decreasing: tannery, textile, and pharmaceuticals load pollutants. Biology, Engineering and Medicine 3(6).
Singh, R L & Singh, R P . 2019. Advances in biological treatment of industrial waste water and their recycling for a sustainable future. Springer
Zhang, Kaiqiang, Suh, Jun Min, Choi, Ji-Won, Jang, Ho Won, Shokouhimehr, Mohammadreza & Varma, Rajender S. . 2019. Recent Advances in the Nanocatalyst-Assisted NaBH4 Reduction of Nitroaromatics in Water. ACS Omega 4(1):483–495.
Feng, Yao, Yin, Juanjuan, Liu, Shufeng, Wang, Yuying, Li, Bingfan & Jiao, Tifeng . 2020. Facile Synthesis of Ag/Pd Nanoparticle-Loaded Poly(ethylene imine) Composite Hydrogels with Highly Efficient Catalytic Reduction of 4-Nitrophenol. ACS Omega 5(7):3725–3733.
Zahoor, Awan, Jeon, Jeong Sook, Jang, Ho Saeng, Christy, Maria, Hwang, Yunju & Nahm, Kee Suk . 2014. Mechanistic Study on Phase and Morphology Conversion of MnO2 Nanostructures Grown by Controlled Hydrothermal Synthesis. Science of Advanced Materials 6(12):2712–2723.
Ullah, Ata, Rahman, Lutfur, Hussain, Syed Zajif, Abbas, Wasim, Tawab, Abdul, Jilani, Asim, Bajwa, Sadia Zafar, Khan, Waheed S., Riaz, Rabia, Hussain, Irshad & Rehman, Asma . 2021. Mechanistic insight of dye degradation using TiO2 anchored α-MnO2 nanorods as promising sunlight driven photocatalyst. Materials Science and Engineering: B 271:115257.
Lan, Binbin, Huang, Shenggen, Ye, Changjing, Qin, Qingqing, Yan, Jian & Wu, Yucheng . 2019. Enhanced electrochemical performance of Sn-doped MnO2 and study on morphology evolution. Journal of Alloys and Compounds 788:302–310.
Nisha, K.D., Navaneethan, M., Dhanalakshmi, B., Hayakawa, Y., Ponnusamy, S. & Muthamizhchelvan, C. . 2013. Formation and morphological investigation of petal-like cadmium sulphide nanostructures. Optical Materials 35(9):1652–1658.
Moulder, J F . 1995. Handbook of X-Ray Photoelectron Spectroscopy. Physical Electronics 230–232.
Xu, Chengjun, Shi, Shan, Sun, Yige, Chen, Yanyi & Kang, Feiyu . 2013. Ultrathin amorphous manganese dioxide nanosheets synthesized with controllable width. Chemical Communications 49(66):7331.
Gubbala, Suresh, Russell, Harry B., Shah, Hemant, Deb, Biswapriya, Jasinski, Jacek, Rypkema, Heather & Sunkara, Mahendra K. . 2009. Surface properties of SnO2 nanowires for enhanced performance with dye-sensitized solar cells. Energy & Environmental Science 2(12):1302.
Jilani, Asim, Rehman, Ghani Ur, Ansari, Mohammad Omaish, Othman, Mohd Hafiz Dzarfan, Hussain, Syed Zajif, Dustgeer, Mohsin Raza & Darwesh, Reem . 2020. Sulfonated polyaniline-encapsulated graphene@graphitic carbon nitride nanocomposites for significantly enhanced photocatalytic degradation of phenol: a mechanistic study. New Journal of Chemistry 44(45):19570–19580.
Raza, Waseem & Krupanidhi, S. B. . 2018. Engineering Defects in Graphene Oxide for Selective Ammonia and Enzyme-Free Glucose Sensing and Excellent Catalytic Performance for para-Nitrophenol Reduction. ACS Applied Materials & Interfaces 10(30):25285–25294.
Panimalar, S., Subash, M., Chandrasekar, M., Uthrakumar, R., Inmozhi, C., Al-Onazi, Wedad A., Al-Mohaimeed, Amal M., Chen, Tse-Wei, Kennedy, J., Maaza, M. & Kaviyarasu, K. . 2022. Reproducibility and long-term stability of Sn doped MnO2 nanostructures: Practical photocatalytic systems and wastewater treatment applications. Chemosphere 293:133646.
Kohantorabi, Mona & Gholami, Mohammad Reza . 2017. Kinetic Analysis of the Reduction of 4-Nitrophenol Catalyzed by CeO2 Nanorods-Supported CuNi Nanoparticles. Industrial & Engineering Chemistry Research. American Chemical Society (ACS) 56:1159–1167 https://dx.doi.org/10.1021/acs.iecr.6b04208
Qi, Bin, Wu, Chenchen, Liu, Yu, Liu, Jun & Zhang, Haibo . 2019. Self-Assembled Magnetic Pt Nanocomposites for the Catalytic Reduction of Nitrophenol. ACS Applied Nano Materials 2(7):4377–4385.
Mohamed, Mohamed Jaffer Sadiq & Denthaje, Krishna Bhat . 2016. Novel RGO-ZnWO4Fe3O4 Nanocomposite as an Efficient Catalyst for Rapid Reduction of 4-Nitrophenol to 4-Aminophenol. Industrial & Engineering Chemistry Research 55(27):7267–7272.
Keywords: Industrial effluent, Carcinogenic, Catalytic reduction, Sn doped MnO2, 4-Nitrophenol
Materials Innovations (MI) is an interdisciplinary journal devoted to significant experimental and theoretical findings on the synthesis, structure, charachterization, processing and applications of materials. Materials Innovations is dedicated to publishing peer reviewed novel, cutting edge reports of broad interest to the materials science community.