
Full Html
Vol 2 Issue 4
Green Synthesis of Copper Oxide Nanoparticles from Papaya/Lemon tea Extract and its Application in Degradation of Methyl Orange
Pages: 115-122
Doi: 10.54738/MI.2022.2401
Doi URL: http://doi.org/10.54738/MI.2022.2401
Ayesha Ikram 1 , Sidra Jamil?? 2 , Muhammad Fasehullah 3
1 Department of Physics, NED University of Engineering & Technology, Karachi, 75270, Pakistan
2 Chongqing Key Laboratory for Advanced Materials and Technologies of Clean Energies, School of Materials and Energy, Southwest University, Chongqing, 400715, PR China
3 State Key Laboratory of Power Transmission Equipment & System Security and New Technology, Chongqing University, Chongqing, 400044, China
Environmental contamination is one of the major and most urgent problems of the modem world. Industries are the greatest polluters, with the textile industry generating high liquid effluent pollutants due to the large amounts of water applied in textile processing. The lowest quantity of synthetic dyes in water can bear upon the aesthetic merit, transparency and gas solubility of water bodies. Nano-metal plays a significant role to minimize the toxicity of water waste due to synthetic dyes. In metal nanoparticles, copper nanoparticles show great catalytic activity. In the present work, degradation of methyl orange would be done by copper oxide nanoparticles. The copper oxide nanoparticles were synthesized by green reduction method using papaya extract and lemon tea extract as a stabilizer. The crystal structure of copper oxide nanoparticles was analyzed by X-Ray diffraction, and SEM was used to determine the particle morphology of nanoparticles.
Keywords
Methyl Orange, Papaya, Lemon Tea, Copper Oxide, Degradation
Nanoparticles are the subject of dynamic studies in the field of research and also have the potential to increase the strength and homogeneity of composite materials. Organic and inorganic nanoparticles are extensively studies in the fields of photochemistry, and electrochemistry.1
Metal nanostructures can be forged in different sizes and configurations
2 , and show a diverse range of applications in the areas of catalysis, electronics, sensors and optical devices.3 It is also gradually becoming essential to use nano-size metal structures as a heterogeneous recyclable catalysts in different environmental problems connected with hazardous wastes and toxic water pollutants.4 Dyes play an essential role in synthetic organic compounds used in many industries, mostly textiles. Nevertheless, these synthetic dyes are highly toxic, carcinogens and mutagens. Industries directly release dyes containing wastewater into adjacent water bodies, such as rivers, lakes, drains, etc.

Figure 1: (a) Systematic Illustration of copper oxide nanoparticles by Papaya/Lemon tea extracts. (b) Schematics of the synthesis procedure

Figure 2: (a, b) Spherical morphology of particles as observed in the SEM
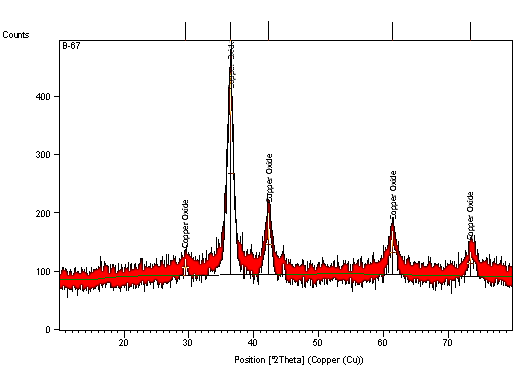
Figure 3: XRD spectrum of copper oxide nanoparticles
Furthermore, in some cases, the wastewater produced by the various industries is used directly for irrigation purposes. This practice deteriorates the quality of the crops and the soil. Consequently, they have become common industrial environmental pollutants.5, 6 It has been estimated that approximately 10-15% of dyes are released into the atmosphere generally through the water. It is recognized that some azo dyes and their breakdown products, such as aromatic amines, are highly poisonous. It has been stated that the release of the same minor amount of dyes into the water interferes with the penetration of light into the water systems. Dyes absorb and reflect sunlight, thereby influencing photosynthesis. Reduced light penetration also influences the solubility of gases. As a result, the quality of receiving waterways has been damaged and may be fatal to aquatic life, food chain organisms and treatment processes.7
Copper/copper oxide nanoparticles hold catalytic activity on a large scale compared to nanoparticles of other materials. Titanium dioxide nanoparticles possess excellent catalytic activity,8 and their activity is enhanced by doping them with copper nanoparticles.9 Reductive devastation of carbon-based contaminants is one of the major threats to the environment; iron is one of the reducing agents in this regard, but the passivity is the disadvantage when using metallic iron, so copper nanoparticles and oxides of copper, when incorporated with iron reduces its passivity10 . Copper oxide particulates show a reduction in methyl-orange concentration. As methyl orange is an organic pollutant, copper and its oxides can play an essential role in its degradation.
There are several methods by which copper/copper oxide nanoparticles can be synthesized, such as vapour deposition, electrochemical reduction11 , thermal decomposition, polyol processes, and laser ablation12 . Sputter deposition, chemical reduction methods and green reduction routes13 . Of all synthetic pathways, many studies have been reported on the green reduction method with antibacterial, neuroprotective, anticancer and photocatalytic activity over the last few years. Green synthesis aims to prevent the production of environmentally harmful products through a reliable, sustainable and ecological synthesis process. The green reduction method of forming metallic nanoparticles is simple, inexpensive, eco-friendly, low energy and non-toxic with a highly stable economic approach and environment-friendly propertie.14 Plant extracts contain a variety of phyto-constituents such as alkaloids, terpenoids, flavonoids, polyphenols, sugars, proteins, etc. with a broad range of reducing capacities acting as reducers and stabilizers during green synthesis.
Papaya (Carica papaya L.) is one of the most common fruits distributed throughout the world. Papaya is a rich source of vitamins including β-carotene, vitamin B (thiamine, riboflavin, niacin and folate), vitamin C, vitamin E, minerals (Na, K, Fe, Ca), and fiber. Papaya juice is consumed for the treatment of various diseases like constipation, dyspepsia, diabetes, cancer, heart stroke, blood pressure, etc.,15 having very beneficial properties, papaya extract may also be used as a stabilizer for nanoparticles.
In current work, copper oxide nanoparticles will be synthesized by green reduction using two different stabilizers (papaya and lemon tea extracts) and will be subsequently used for the degradation of methyl orange.

Figure 4: (a) MO initial concentration (b) addition of hydrazine hydrate in MO (c) Concentration of MO just after the addition of copper oxide NPs by papaya extract (d) concentration of MO just after the addition of copper oxide NPs by lemon-tea extract
Copper (II) sulfate pentahydrate (CuSO4.5H2O), L-ascorbic acid (C6H8O6), sodium hydroxide (NaOH), methanol (CH4O) and deionized water are purchased from Karachi scientific traders. Methylene Orange (MO), Hydrazine Hydrate (H6N2O) 80AR-Malaysian obtained from Karachi chemical services. All chemicals are of lab grade and used without further refining/purification.
Copper (II) sulfate pentahydrate solution with 0.04M was prepared in 100 ml of deionized water. The solutions of L-Ascorbic acid, Sodium hydroxide and hydrazine hydrate with the molarities of 0.001 M, 0.01 M and 1 M, respectively, were prepared by using 100 ml of deionized water, and Methyl orange of 0.001M was prepared by adding 327.34 mg of methyl orange in 1000 ml of deionized water.
Initially, 100 ml Copper (II) sulfate pentahydrate solution (0.04 M) was taken in a clean beaker, and gradually 100 ml of papaya extract & 10 ml of L-ascorbic acid (0.001 M) solutions were added to the beaker under constant normal stirring. The papaya extract solution was used as a stabilizer; moreover, L-Ascorbic acid served as an anti-oxidant agent. A pre-determined quantity of NaOH (0.01 M) was added to adjust the pH to 12. Finally, Hydrazine hydrate (1 M) was added under constant stirring as a reducing agent. The colour change of the solution indicated the completion of the reaction as the dark brown coloured precipitates appeared in the solution. The precipitates were washed several times with de-ionized water and methanol after filtration and then dried, and the required powder was obtained. Copper oxide nanoparticles were also synthesized using lemon tea extract following the same procedure.
The initial concentration of the dye was estimated then a hydrazine hydrate of 1 M was added to the solution. The concentration of MO with hydrazine hydrate was also estimated. Then, a dye solution with hydrazine hydrate was taken out into eight test tubes; each test tube contained a 10 ml solution mixture of dye and hydrazine hydrate. 1 mg of already prepared copper oxide nanoparticles was added to each test tube. In four test tubes, copper oxide nanoparticles synthesized using papaya extract were added and in remaining four test tubes, copper oxide nanoparticles prepared by lemon tea extract were added. Two test tubes were placed in the visible range; two were placed in the dark, and two test tubes in the UV range; all samples were placed for one hour, and each sample set contained one test tube having copper oxide nanoparticles prepared from papaya extract and other test tube having copper oxide nanoparticles synthesized by lemon tea extract. Two test tubes were picked up for the initial estimation of the degradation of methyl orange. The absorbance of dye before and after degradation was found at different wavelengths using a UV-Vis spectrophotometer.
The chemistry and crystal structure of copper oxide nanoparticles was determined by the X-Ray Diffraction (XRD) (XPERT-PRO); coupled with a copper monochromator using Cu K radiation (=1.54060 A) at 40kv, 30mA in the range of 2 thetas from 10-80. Particle size was estimated by Scherer's formula. Moreover, the morphology of copper oxide nanoparticles was investigated using a scanning electron microscope (FEI Quanta 200).
The SEM images of copper oxide nanoparticles synthesized by green reduction method are presented in Figures 2(a & b). SEM results show that the morphology of prepared nanoparticles from a green method is spherical, however, some degree of agglomeration was also evident in the images.
The particle size of copper oxide nanoparticles synthesized by a green method is calculated by equation 1.
D=0.9λ/β Cosθ (1)
Where β is the full width at half the maximum of the X-ray profile, θ is the Bragg angle, and λ is the wavelength of X-rays. Figure 3 shows typical patterns of copper oxide nanoparticles.
Particle size and d-spacing of the diffraction patterns of the copper oxide nanoparticles synthesized by a green method. is 8.85 nm and 2.466, respectively. The full width at half maximum (FWHM) was used to evaluate the particle size of copper oxide nanoparticles. FWHM value was 0.016 radian. The most intense peak of copper oxide nanoparticles is usually used to calculate the particle size to get better results. The most intense XRD peak was at 2θ = 36.42o, corresponding to the (111) plane having a lattice constant of 4.27 Å.
UV-Visible spectrometer with the range of wavelength (350-600) nm was used to analyze the effect of copper oxide nanoparticles on the concentration of methyl orange. MO shows the absorbance peak at 530 nm, as shown in Figure 4(a). For the degradation test, MO of 10-3 M is used. The pace of degradation of methyl orange evaluated from the equation no. 2
a%={Co-CCo*100}
Where C is the absorbance after time t and Co is the initial dye concentration before degradation. As given in Figure 4(b), a decrease in the concentration of about 9.99% was observed when the hydrazine hydrate of 1M was added to the solution. The initial sample was taken without a catalyst.

Figure 5: (a) concentration of MO in the visible range, (b) in the dark, and (c) in UV light by copper oxide nanoparticles stabilized with papaya extract. The samples were placed for one hour.

Figure 6: (a) concentration of MO in the visible range, (b) in the dark, (c) in UV light by copper oxide nanoparticles stabilized by the lemon-tea extract. The samples were placed for one hour.
Samples were taken to estimate the decrease in absorbance of MO after adding copper oxide nanoparticles; the first sample was collected just after the addition of nanoparticles. The samples were placed in visible light, in the dark, and the ultraviolet range for one hour; there was a sudden decrease in the concentration of dye just after the addition of copper oxide nanoparticles stabilized by papaya extract and lemon tea extract by 14.87% and 17.57% as shown in Figures 4(c & d) respectively.
The samples that were placed in visible light, in the dark and in the UV light for an hour show a decrease in the concentration of MO by 18.45 %, 17.61 % and 30.98 %, as shown in Figures 5 (a, b) and (c) respectively when the copper oxide nanoparticles via papaya extract were used as a catalyst. Even in dark conditions, copper oxide nanoparticles possess a catalytic effect as the concentration of MO decreases by 17.61%.
When copper oxide nanoparticles, which were synthesized by using lemon tea extract, were used as a catalyst for the degradation of dye, the concentration of MO decreased by 31.95%, 27.21 % and 45.23 % of the samples which were placed in visible light, in the dark and in the UV light as presented in Figures 6(a-c) correspondingly. Above results shows that the catalytic effect of the sample for copper oxide nanoparticles prepared with lemon tea extract illustrates better catalytic activity than the one synthesized with papaya extract.
From results, it has been observed that for both stabilizers (papaya/lemon tea extract) in UV light the phenomena of degradation promoted this happened due to the reason that in the presence of catalyst the reaction is accelerated by the absorption of photon with the energy that should be equal or higher than the band-gap energy of the catalyst. Due to the absorption of photon, there is a transition of electron from the valence band of the semiconductor catalyst to the conduction band, thus generating a hole in the valence band. These activated electrons react with an oxidants of dye to produce a reduced product.
Copper oxide nanoparticles were synthesized via the green reduction method using papaya extract/ lemon tea extract as a stabilizer. SEM images reveal spherical morphology with a particle size of 8.85 nm, as calculated from the XRD analysis. From the results of methyl orange degradation, it can be clearly observed that the copper oxide nanoparticles stabilized by the lemon tea extract show greater catalytic activity compared with nanoparticles stabilized by papaya extract. It has also been clearly observed that the samples placed in the ultraviolet light show improved catalytic activity compared with the samples placed in a dark and visible range. From observations, it can be evidently analyzed that the copper oxide nanoparticles possess a catalytic property even in the dark. Hence, the environmentally friendly route of the synthesis of copper oxide nanoparticles and its outstanding catalytic activity provide insight for widespread applications.
Grateful to the NED University of Engineering and Technology for the required assistance and facilities.
Sandeep, Shadakshari S, Santhosh, Arehalli S., Swamy, Ningappa S Kumara, Suresh, Gurukar S., Melo, Jose S. & Mallu, Puttaswamappa . 2016. Biosynthesis Of Silver Nanoparticles Using Convolvulus Pluricaulis Leaf Extract And Assessment Of Their Catalytic, Electrocatalytic And Phenol Remediation Properties. Advanced Materials Letters 7(5):383–389.
Fasehullah, Muhammad, Wang, Feipeng, Jamil, Sidra, Huang, Jingliang, Haq, Inzamam Ul & Han, Qiuhuang . 2021. Effect of SiO2 Nanoparticle's Size and Doping Concentration on AC Breakdown Behavior of Insulating Oil-based Nanofluids. 2021 IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP) 363–366.
Fasehullah, Muhammad, Wang, Feipeng & Jamil, Sidra . 2022. Significantly elevated AC dielectric strength of synthetic ester oil-based nanofluids by varying morphology of CdS nano-additives. Journal of Molecular Liquids 353:118817.
Soomro, Razium Ali, Nafady, Ayman, Sirajuddin, , Sherazi, Syed Tufail Hussain, Kalwar, Nazar Hussain, Shah, Mohammad Raza & Hallam, Keith Richard . 2015. Catalytic Reductive Degradation of Methyl Orange Using Air Resilient Copper Nanostructures. Journal of Nanomaterials 2015:1–12.
Gola, Deepak, Kriti, Anu, Bhatt, Neha, Bajpai, Medha, Singh, Astha, Arya, Arvind, Chauhan, Nitin, Srivastava, Sunil Kumar, Tyagi, Pankaj Kumar & Agrawal, Yamini . 2021. Silver nanoparticles for enhanced dye degradation. Current Research in Green and Sustainable Chemistry 4:100132.
Su, Yuanjie, Yang, Ya, Zhang, Hulin, Xie, Yannan, Wu, Zhiming, Jiang, Yadong, Fukata, Naoki, Bando, Yoshio & Wang, Zhong Lin . 2013. Enhanced photodegradation of methyl orange with TiO2 nanoparticles using a triboelectric nanogenerator. Nanotechnology 24(29):295401.
Lavanya, C, Dhankar, Rajesh, chhikara., Sunil & Sheoran, Sarita . 2014. Degradation of Toxic Dyes: A Review. International Journal of Current Microbial Applied Science 3:189–199.
Jamil, Sidra & Fasehullah, Muhammad . 2021. Effect of Temperature on Structure, Morphology, and Optical Properties of TiO2 Nanoparticles. Materials Innovations 01(01):22–28.
An, J & Zhou, Q . 2012. Degradation of Some Typical Pharmaceuticals and Personal Care Products with Copper-Plating Iron Doped cu2o under Visible Light Irradiation. Journal of Environmental Sciences 24:827–833.
Yang, Xi-Jia, Wang, Shu, Sun, Hai-Ming, Wang, Xiao-Bing & Lian, Jian-She . 2015. Preparation and photocatalytic performance of Cu-doped TiO2 nanoparticles. Transactions of Nonferrous Metals Society of China 25(2):504–509.
Yang, Xuegeng, Chen, Shenhao, Zhao, Shiyong, Li, Degang & Ma, Houyi . 2003. Synthesis of copper nanorods using electrochemical methods. Journal of the Serbian Chemical Society 68(11):843–847.
Jaehoon, L, Dong, K.-K & Weekyong, K . 2006. Preparation of Cu Nanoparticles from Cu Powder Dispersed in 2-Propanol by Laser Ablation. Bulletin of the Korean Chemical Society 27:1869–1872.
Annapurna, Sathiraju, Suresh, Yathapu, Sreedhar, Bojja, Bhikshamaiah, Ganghishetti & Singh, A K . 2014. Characterization of Green Synthesized Copper Nanoparticles Stabilized by Ocimum Leaf Extract. MRS Proceedings 1704:1704.
Kim, Beomjin, Song, Woo Chang, Park, Sun Young & Park, Geuntae Y . 2021. Green Synthesis of Silver and Gold Nanoparticles via Sargassum serratifolium Extract for Catalytic Reduction of Organic Dyes. Catalysts 11(3):347.
Phang, You-Kang, Aminuzzaman, Mohammod, Akhtaruzzaman, M D, Muhammad, Ghulam, Ogawa, Sayaka, Watanabe, Akira & Tey, Lai-Hock . Green Synthesis and Characterization of CuO Nanoparticles Derived from Papaya Peel Extract for the Photocatalytic Degradation of Palm Oil Mill Effluent (POME) Sustainability 13(2):796.
Keywords: Methyl Orange, Papaya, Lemon Tea, Copper Oxide, Degradation
Materials Innovations (MI) is an interdisciplinary journal devoted to significant experimental and theoretical findings on the synthesis, structure, charachterization, processing and applications of materials. Materials Innovations is dedicated to publishing peer reviewed novel, cutting edge reports of broad interest to the materials science community.